Introduction
Lung cancer is the second most common cancer in the world and the fourth most common cancer in Korea. In Korea, 23,177 patients were newly diagnosed in 2013 and 17,330 lung cancer patients died in 2014 [
1]. Lung cancer has been the leading cause of cancer death in Korea since 1983. The prevalence of lung cancer is still increasing and expected to continue increasing for the next 10 years [
2].
In lung cancer, radiotherapy (RT) plays a major role in treatment with curative intent [
3,
4]. However, the 5-year loco-regional progression rate of non-small cell lung cancer (NSCLC) with initial concurrent RT treatment is 28.9% [
5], and local failure of small cell lung cancer (SCLC) treatment occurs in up to 36%-52% of patients with initial concurrent RT [
6]. Locoregional disease recurrence continues to be a major problem and has been the main cause of death after initial definitive chemoradiotherapy or RT.
Since there are few prospective trials for high-dose re-irradiation (re-RT) in the thorax, the effect and safety of high dose re-RT is uncertain. High-dose re-RT might cause severe radiation injury, such as radiation pneumonitis, esophagitis, fistula, and stenosis. Furthermore, the tolerance of normal tissues in the context of re-RT is unknown. However, owing to recent technological advances in RT and imaging techniques, high-dose re-RT is now considered to be a potential salvage treatment approach [
7-
9].
In this study, we retrospectively analyzed our institutional experience to evaluate cancer control parameters, including local control (LC), loco-regional progression-free survival (LRFS), local progression-free survival (LPFS), and overall survival (OS), as well as the toxicities of re-RT using highly conformal RT, such as intensity modulation radiotherapy (IMRT) or stereotactic body radiotherapy (SBRT).
Go to :

Materials and Methods
1. Patients
From August 2005 to November 2016, 56 patients with lung cancer received a second course of thoracic RT. Each patient was discussed in a multidisciplinary team with thoracic surgeons, respiratory physicians, radiologists and medical oncologist before they decided to receive a second course of RT. Patients who satisfied the following criteria were included in the study: (1) the primary tumor was histologically diagnosed as NSCLC or SCLC; (2) recurrences were confirmed by documented radiographic findings and/or pathological biopsies within the thoracic area; (3) patients had received re-RT to the thorax; (4) patients had received curative intent RT of more than 50 Gy for NSCLC and for patients with SCLC who were treated with radical concurrent chemoradiation (CCRT), with 45 Gy twice a day; and (5) there were no active distant metastasis or controlled solitary distant metastasis at the time of re-RT.
Patient charts were reviewed, and the following data were collected: sex, smoking history, surgery history, history of chemotherapy, Eastern Cooperative Oncology Group (ECOG) performance status (PS) before re-RT, tumor histology, stages at initial RT and second RT, planning tumor volume (PTV), and gross tumor volume (GTV) at the first and second RT (mL), RT doses, biologically equivalent dose (BED, α/β=10 and 3), fraction size of at the first and second RT, RT techniques of the initial course and of re-RT, time interval between RT courses, acute and chronic toxicities, survival duration, LC, LRFS, LPFS, and distant metastasis-free survival (DMFS). All of the tumors were staged using the 7th edition of the American Joint Committee on Cancer (AJCC) staging.
2. Treatment/radiotherapy
For treatment planning, pretreatment imaging was obtained in the supine position. For the re-RT, 4-dimensional computed tomography (4D-CT) was taken routinely. The primary tumor and enlarged lymph nodes were contoured as the GTV on axial treatment planning CT based on the pretreatment chest CT and/or fluorine-18-2-fluoro-2-deoxy-D-glucose positron emission tomography and computed tomography (FDG-PET/CT). The clinical target volume (CTV) was defined as a 0.5 cm expansion from the GTV, and the PTV was determined by expanding the CTV by adequate margins from the tumor movement assessed in 4D-CT.
The treatments were delivered using the three-dimensional conformal radiotherapy (3D-CRT) technique with a linear accelerator for the initial RT, except for four patients who were treated using IMRT technique. For re-RT, every treatment was administered using daily volumetric image-guidance RT using kilovoltage or megavoltage cone beam CT from a highly conformal treatment modality, such as IMRT or helical tomotherapy (TOMO). A noncoplanar field technique was used for IMRT or SBRT. When patients have lesions close proximity to trachea or esophagus, to spare the region of interests more efficiently, TOMO were used for a re-RT plan.
3. Follow-up and toxicity analysis
After the re-RT was completed, patients were regularly followed up at 2-3 month intervals with examinations, such as blood tests, chest X-rays, chest computed tomography (CT), and FDG-PET/CT if needed. LC was defined as no progressive disease in the high-dose treatment volume within 50% of the isodose line.
The OS time after re-RT was the time between the first day of the second RT and the date of the patient’s death or most recent follow-up visit. LPFS was defined as the time between the first day of the second RT and date of recurrence in the re-irradiated high-dose treatment volume. LRFS and DMFS were defined as the time between the first day of the second RT and the date that the loco-regional recurrence or distant metastasis was documented and imaged.
The severity of the acute and late toxicities was evaluated by the Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.03. Acute toxicities were defined as adverse effects that appeared within 3 months from the start of RT. The adverse effects that appeared after three months were defined as late toxicities.
4. Statistical analysis
Survival outcomes were estimated by Kaplan-Meier analysis. Mann-Whitney analysis was used to evaluate the relationship between the RT parameters and toxicities. Cox regression was used to assess the associations between the survival outcomes and predictive factors. A p-value of < 0.05 was considered significant. Multivariable analysis was precluded by the small number of events. All statistical analyses were performed with SPSS software ver. 23.0 (IBM Corp., Armonk, NY).
5. Ethical statement
This study was approved by the Institutional Review Board (IRB) of Seoul St. Mary's Hospital (IRB number: KC17RESI0660). The necessity of informed consent for this retrospective analysis was waived.
Go to :

Results
1. Patient and tumor characteristics
Thirty-one patients, among which 24 (77.4%) had NSCLC and seven (22.6%) had SCLC, were included in the second course of thoracic re-RT. The patient characteristics are shown in
Table 1. The median age at the re-RT was 63.6 years (range, 43.6 to 88.9 years). There were 27 males (87.1%) and four females (12.9%). Twenty-nine patients (93.5%) showed an ECOG PS 0 or 1 at the time of re-RT. Only two patients showed an ECOG 2.
Table 1.
Characteristic of patients and tumors
|
Characteristic |
Value |
|
Age at re-RT (yr)
|
63.6 (43.6-88.9) |
|
Sex
|
|
|
Male |
27 (87.1) |
|
Female |
4 (12.9) |
|
History of tobacco use
|
25 (80.6) |
|
History of thoracic surgery
|
9 (29.0) |
|
ECOG PS at re-RT
|
|
|
0 |
6 (19.4) |
|
1 |
23 (74.2) |
|
2 |
2 (6.5) |
|
Histology
|
|
|
NSCLC |
|
|
SqCC |
15 (48.4) |
|
Adenoca |
8 (25.8) |
|
Others, large cell |
1 (3.2) |
|
SCLC |
7 (22.6) |
|
Initial AJCC stage
|
|
|
NSCLC |
|
|
I |
3 (9.7) |
|
II |
4 (13) |
|
III |
14 (45.1) |
|
IV |
2 (6.4) |
|
Unknown |
1 (3.2) |
|
SCLC |
|
|
LD |
5 (16.1) |
|
ED |
2 (6.5) |

In nine cases (29%), pathological confirmation of recurrence or progression was performed before re-RT. One patient with squamous cell carcinoma at the initial treatment was confirmed as having a non-specified malignancy, and one patient with SCLC at the initial diagnosis was shown to have squamous cell carcinoma with neuroendocrine differentiation. Except for these two patients, the pathologic results were the same as the initial results. Three patients were given CCRT as re-RT because PTV was small as they recurred on a single lymph node and they weren’t heavily treated with chemotherapy. Four SCLC patients were treated with chemotherapy after re-RT as courses of treatment. For NSCLC patients, ten patients hadn’t received treatment including radiation, surgery, and chemotherapy until progressions of disease had found. However, after the progressions have found, chemotherapy was given. Five NSCLC patients were given chemotherapy after re-RT as courses of the treatment because their recurred tumors burden was large with the nodal chain metastasis or large size of the recurrent tumor. Therefore, nineteen patients (61.3%) received additional systemic chemotherapy after re-RT.
The overview of the initial RT and re-RT is shown in
Tables 2 and
3. The median interval between the end of the initial RT and the start of re-RT was 15.1 months (range, 4.4 to 56.3 months). For the initial RT, the median RT dose was 60 Gy (range, 45 to 66 Gy) and 79.2 Gy
10 (range, 51.75 to 150 Gy
10). Twenty-six patients (83.9%) received RT via 3D-CRT, and four patients were treated with linac–based IMRT or TOMO. Concurrent chemotherapy was delivered to 19 patients (61.3%). The median PTV was 353.15 mL (range, 33.3 to 679.9 mL) and the median GTV was 57.4 mL (range, 2.83 to 211.5 mL).
Table 2.
Overview of the initial RT
|
Parameter |
Value |
|
Dose (Gy)
|
60 (45-66) |
|
NSCLC |
64.5 (50-66) |
|
SCLC |
45 |
|
Dose, BED Gy10
|
79.2 (51.8-150) |
|
NSCLC |
79.2 (60-150) |
|
SCLC |
51.8 |
|
Fraction size
|
2 (1.5-15) |
|
Type of radiation
|
|
|
Conformal |
27 (87.1) |
|
IMRT |
4 (12.9) |
|
Concurrent chemotherapy
|
|
|
Yes |
19 (61.3) |
|
No |
12 (38.7) |
|
RT purpose
|
|
|
Radical |
21 (67.7) |
|
Salvage |
5 (16.1) |
|
Adjuvant |
5 (16.1) |
|
PTV (mL)
|
353.2 (33.3-679.9) |
|
GTV (mL)
|
57.4 (2.8-211.5) |

Table 3.
|
Characteristic |
Value |
|
Interval to re-RT
|
15.1 (4.4-56.3) |
|
Dose (Gy)
|
50 (35-65) |
|
NSCLC |
51.4 (45-65) |
|
SCLC |
45 (35-55) |
|
Dose, BED Gy10
|
68.8 (43.2-132) |
|
NSCLC |
68.8 (55.1-132) |
|
SCLC |
58.5 (43.2-70.2) |
|
Fraction size (Gy)
|
|
|
≤ 3 |
21 (67.7) |
|
> 3 |
10 (32.2) |
|
Re-RT field according to initial RT field (70% dose line level)
|
|
|
In-field |
23 (74.2) |
|
Out-field |
8 (25.8) |
|
Laterality according to initial RT field
|
|
|
Ipsilateral |
25 (80.6) |
|
Contralateral |
6 (19.4) |
|
Type of radiation
|
|
|
SBRT |
10 (32.3) |
|
IMRT, TOMO |
21 (67.7) |
|
Concurrent chemotherapy
|
|
|
Yes |
3 (9.7) |
|
No |
28 (90.3) |
|
Median PTV (mL)
|
51.3 (13-299.3) |
|
Median GTV (mL)
|
13.5 (1.4-124.9) |

For re-RT, the median RT dose was 50 Gy (range, 35 to 65 Gy) and 68.8 Gy10 (range, 43.2 to 132 Gy10). All patients were treated using high conformal RT via IMRT or SBRT. Only three patients (9.7%) were administered concurrent chemotherapy. Ten patients (32.2%) showed more than 3 Gy in the RT fraction size. By a multidisciplinary team, elderly patients, patients with comorbidities, and patients with poor pulmonary function tests tend to decide to treat with radiation, especially SBRT rather than surgery. The median PTV was 51.3 mL (range, 13 to 299.3 mL), and the median GTV was 13.45 mL (range, 1.4 to 124.9 mL).
2. Treatment outcomes and survival
At the time of the last follow up, 23 patients were deceased and eight patients were alive. The median follow-up time was 17.4 months (range, 4.8 to 76.8 months). Eighteen patients (58.1%) showed loco-regional recurrence, sixteen exhibited progression in the high-dose irradiated volume and two patients had progression of the regional nodal chain in the out-field. Eleven patients (35.5%) showed distant metastasis after re-RT. Both local recurrence and distant metastasis after re-RT were observed in six patients. The major sites of distant metastasis were the brain and the contra-lateral lung, which occurred in four patients (36.4%) each. Abdominal lymph node metastasis occurred in two patients (18.2%), while hepatic metastasis occurred in one patient (9.1%), and bone metastasis occurred in one patient (9.1%).
The crude LC was 48.4%. The 1-year and 2-year LC rates were 60.2% and 43.7%, respectively. The LRFS was 15.4 months (range, 3.4 to 76.8 months). 1-Year and 2-year LRFS rates were 58.1%, and 36.2%, respectively (
Fig. 1). The median DMFS was 29.8 months (range, 2.2 to 76.8 months). 1-Year and 2-year DMFS rates were 72.8% and 65.5%, respectively. The median OS was 20.4 months (range, 4.8 to 76.8 months). 1-Year and 2-year OS rates were 76.8% and 39.4%, respectively (
Fig. 2).
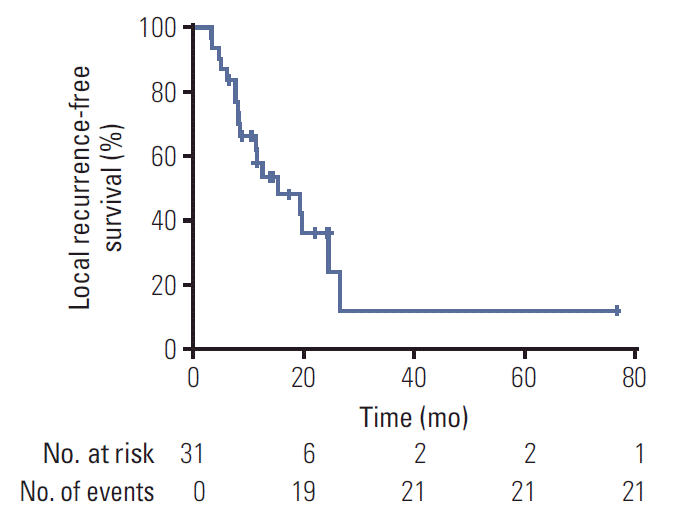 | Fig. 1.Kaplan-Meier curve of local recurrence-free survival for all patients. 
|
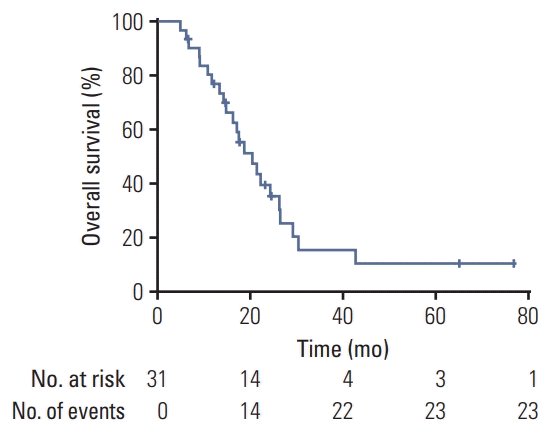 | Fig. 2.Kaplan-Meier curve of overall survival for all patients. 
|
According to univariate analysis, the cumulative BED
10 ≥ 145 Gy
10 and re-RT BED
10 ≥ 68.7 Gy
10 were significant factors for a longer OS (p=0.029 and p=0.012, respectively). They were also associated with a longer LRFS (p=0.003 and p=0.000, respectively). Higher cumulative BED
10 and re-RT BED
10 also typically showed trends toward longer DMFS (p=0.072 and p=0.053, respectively). These findings demonstrate that a higher radiation dose leads to a better treatment outcome in locoregionally recurrent lung cancer. Chemotherapy after re-RT, male gender, PTV < 51.3 mL at re-RT, GTV < 10 mL at re-RT and fraction size > 3 Gy were also significant factors for longer DMFS (p=0.020, p=0.034 p=0.007, p=0.024, and p=0.006, respectively). In those small volume tumors, high-dose radiation to recurrent lung cancer resulted in a long DMFS. There were no significant differences in the OS, LRFS, and DMFS with regard to the other factors (
Table 4).
Table 4.
Univariate analysis of clinical and dosimetric factors’ effect on OS, LRFS, and DMFS
|
Variable |
No. |
OS
|
LRFS
|
DMFS
|
|
Median OS |
p-value |
Median LRFS |
p-value |
Median DMFS |
p-value |
|
Sex
|
|
|
|
|
|
|
|
|
Male |
27 |
22.1 |
0.085 |
19.3 |
0.933 |
64.3 |
0.034 |
|
Female |
4 |
16.2 |
|
15.4 |
|
5.6 |
|
|
Age at re-RT (yr)
|
|
|
|
|
|
|
|
|
≥ 60 |
23 |
20.4 |
0.715 |
15.4 |
0.999 |
29.8 |
0.858 |
|
< 60 |
8 |
17.4 |
|
12.6 |
|
22.0 |
|
|
Smoking history
|
|
|
|
|
|
|
|
|
Neversmoker |
2 |
14.7 |
0.800 |
6.2 |
0.610 |
- |
0.317 |
|
Ex-smoker/Smoker |
25 |
21.3 |
|
19.3 |
|
17.0 |
|
|
Pathology
|
|
|
|
|
|
|
|
|
NSCLC |
24 |
21.3 |
0.407 |
19.3 |
0.273 |
64.3 |
0.132 |
|
SCLC |
7 |
17.0 |
|
15.4 |
|
29.8 |
|
|
N stage
|
|
|
|
|
|
|
|
|
N0 |
6 |
21.3 |
0.717 |
- |
0.144 |
- |
0.132 |
|
N+ |
21 |
17.4 |
|
12.6 |
|
17.0 |
|
|
Chemotherapy after re-RT
|
|
|
|
|
|
|
|
|
Yes |
19 |
21.3 |
0.717 |
11.5 |
0.137 |
29.8 |
0.020 |
|
No |
12 |
20.4 |
|
24.6 |
|
- |
|
|
Comorbidity
|
|
|
|
|
|
|
|
|
Yes |
18 |
17.0 |
0.485 |
19.3 |
0.931 |
64.3 |
0.963 |
|
No |
13 |
20.4 |
|
15.4 |
|
29.8 |
|
|
CCRT at re-RT
|
|
|
|
|
|
|
|
|
Yes |
3 |
20.4 |
0.551 |
8.3 |
0.807 |
64.3 |
0.543 |
|
No |
28 |
18.6 |
|
15.4 |
|
29.8 |
|
|
Interval between initial RT and re-RT
|
|
|
|
|
|
|
|
|
< 15.1 |
15 |
18.6 |
0.601 |
12.6 |
0.170 |
64.3 |
0.724 |
|
≥ 15.1 |
16 |
20.4 |
|
24.6 |
|
29.8 |
|
|
Total BED10
|
|
|
|
|
|
|
|
|
≥ 145 |
12 |
22.1 |
0.029 |
26.6 |
0.003 |
64.3 |
0.072 |
|
< 145 |
19 |
17 |
|
11.4 |
|
29.8 |
|
|
Re-RT BED10
|
|
|
|
|
|
|
|
|
≥ 68.7 |
16 |
24.2 |
0.012 |
24.6 |
0.000 |
64.3 |
0.053 |
|
< 68.7 |
15 |
16.2 |
|
8.4 |
|
22.0 |
|
|
GTV at re-RT (mL)
|
|
|
|
|
|
|
|
|
≥ 10 |
16 |
17.4 |
0.936 |
11.5 |
0.680 |
29.8 |
0.024 |
|
< 10 |
12 |
20.4 |
|
15.4 |
|
- |
|
|
PTV at re-RT (mL)
|
|
|
|
|
|
|
|
|
≥ 51.3 |
15 |
17.4 |
0.551 |
19.3 |
0.620 |
29.8 |
0.007 |
|
< 51.3 |
14 |
20.4 |
|
12.6 |
|
- |
|
|
Fraction size (Gy)
|
|
|
|
|
|
|
|
|
≤ 3 |
21 |
17.0 |
0.094 |
11.5 |
0.081 |
13.3 |
0.006 |
|
> 3 |
10 |
22.1 |
|
24.6 |
|
- |
|

3. Toxicity
The most frequent acute toxicity was grade 1-2 pulmonary toxicity including dyspnea, cough and pneumonitis. No grade 3-4 acute toxicities were observed. Grade 1 and 2 pulmonary toxicities were observed in 18 and 10 patients, respectively, at a median of 3.02 months (range, 0.17 to 13.9 months). The median cumulative mean lung dose (MLD) was 16.28 Gy
3. There was a statistically significant difference in the cumulative MLD and acute pulmonary symptoms (p=0.004). There were no statistical differences in pulmonary toxicities associated with the total BED
10 dose, re-RT BED
10 dose, re-RT PTV volume, or location of the RT field, which were either central or peripheral and overlapped volume the prior RT field (
Table 5).
Table 5.
Acute and chronic toxicities (CTCAE ver. 4.03)
|
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
|
Acute toxicities
|
|
|
|
|
|
Esophagitis |
4 (12.9) |
0 |
0 |
0 |
|
Pericarditis |
2 (6.5) |
0 |
0 |
0 |
|
Pulmonary (dyspnea, cough, pneumonitis) |
7 (22.6) |
6 (19.4) |
0 |
0 |
|
Chest wall pain |
2 (6.5) |
3 (9.7) |
0 |
0 |
|
Radiation dermatitis |
2 (6.5) |
0 |
0 |
0 |
|
Chronic toxicities
|
|
|
|
|
|
Esophagitis |
1 (3.2) |
1 (3.2) |
0 |
0 |
|
Pericarditis |
3 (9.7) |
0 |
1 (3.2) |
0 |
|
Pulmonary (dyspnea, cough, pneumonitis) |
11 (35.5) |
4 (12.9) |
0 |
0 |
|
Chest wall pain |
1 (3.2) |
1 (3.2) |
0 |
0 |

Grade 1 and 2 esophagitis was observed in five and one patients, respectively. The median cumulative maximum esophagus dose was 124.0 Gy3, and there were no statistically significant factors that predicted esophagitis, including the cumulative maximum esophagus dose, cumulative mean esophagus dose, overlapped portion of the RT field and tumor location.
Other grade 2 toxicities, including acute or chronic chest wall pain were observed in four patients and chronic pericarditis in one patient. One patient required pericardiocentesis two years after re-RT, and he also had coronary angiography (CAG) with percutaneous coronary intervention (PCI) due to underlying cardiac artery disease. There were no grade 4 or 5 cardiac events observed.
Go to :

Discussion
In this study, the outcomes of 31 lung cancer patients who received more than two courses of radiation therapy in the thorax were reviewed retrospectively. The study showed promising survival outcomes after thoracic re-RT for lung cancer with a median OS of 20.4 months and a 1-year OS of 76.8%. These results compare favorably with previous studies of thoracic re-RT (14-18 months and 47%-59%, respectively) (
Table 6) [
10-
15]. The statistically significant prognostic factors for longer OS were a cumulative BED
10 dose ≥ 145 Gy
10 and re-RT BED
10 dose≥ 68.7 Gy
10. Reyngold et al. [
13] and McAvoy et al. [
14] also reported a significant relationship between a higher re-RT dose and longer OS. In several studies, longer interval between the initial RT and re-RT [
7,
16,
17], smaller PTV [
11-
13,
18] and PS before re-RT [
7,
13,
19] were reported as being significant factors associated with improved OS. In this study, none of these factors showed significant differences. However, smaller GTV < 10 mL and PTV < 51.3 mL in re-RT showed trends toward longer OS, even if the trends were not statistically significant. Compared with the previous studies, the patients in this study tended to have relatively small PTV values with a median PTV value of 51.3 mL. Almost all patients, except two who had an ECOG PS of 2, had a good PS before re-RT. The good PS and small PTV might explain the favorable OS. Based on our results and those of previous studies, to achieve better survival outcomes, patient selection might be crucial.
Table 6.
Summary of survival and toxicities in high-dose re-irradiation studies
|
Study |
No. of patients |
Median (range)
|
6 mo/12 mo/24 mo (%) |
LRFS |
6 mo/12 mo/24 mo (%) |
Toxicity |
|
Follow-up (mo) |
Time interval (mo) |
Initial RT dose (Gy) |
Re-RT dose (Gy) |
OS (mo) |
|
Wu et al. (2003) [10] |
13 radical |
15 (2-37) |
13 (6-42) |
66 (56-78) |
51 (46-60) |
14 (2-37) |
-/59/21 |
Not stated |
-/51/42 |
G1-2 Pneumonitis 22% |
|
G1-2 Esophagitis 9% |
|
Ohguri et al. (2012) [11] |
33 |
11 (2-119) |
7.9 (1.1-28.2) |
70 (30-85) |
50 (29-70) |
18.1 |
Not stated |
12.1 |
Not stated |
G2 Pneumonitis 9% |
|
Griffioen et al. (2014) [12] |
24 |
19.3 (2.8-35.9) |
51 (5-189) |
59.8 (24-70) |
60 (39-66) |
13.5 (5.7-21.2) |
80/51/- |
EFS |
62/37/- |
G1-2 Cough G1-2 41.7% |
|
8.4 (5.5-11.3) |
G1-2 Esophagitis 45.8% |
|
G5 Bleeding 12.5% |
|
Reyngold et al. (2013) [13] |
39 |
12.6 (1.3-47.5) |
37 (1-180) |
61 (30-80) |
BED10 70.4 (42.6-180) |
22 |
Not stated |
13.8 |
-/77/64 |
G2 Lung 18% |
|
G3 Lung 5% |
|
McAvoy et al. (2013) [14] |
33 |
11 (1.4-32.4) |
36 (1-376) |
63 (40-74) |
66 (16.4-75) |
11.1 (1.4-32.4) |
75/47/33 |
18 |
79/54/24 |
G3 Lung 23% |
|
RBE |
G4 Lung 6% |
|
G3 Esophagitis 9 |
|
G4 Esophagitis 3% |
|
Trovo et al. (2014) [21] |
17 |
18 (4-57) |
Not stated |
50-60 |
30/5-6 fractions |
19 |
-/59/29 |
Not stated |
-/88/- |
G3 Lung 23% |
|
G5 Lung 0.5% |
|
G5 Bleeding 0.5% |
|
Peulen et al. (2011) [22] |
29 |
12 (1-97) |
14 (5-54) |
BED10 75 (35-112.5) |
BED10 75 (57.6-112.5) |
19 (1.1-98.6) |
-/52/43 |
Not stated |
-/59/- |
G4 Fistula, stenosis 3.4% |
|
G5 Bleeding 10% |
|
Sumita et al. (2016) [18] |
21 |
22.1 (2.3-56.4) |
26.8 (11.4-92.3) |
NSCLC: 60 Gy10 (51.8-87.5), SCLC: 50 Gy10 (50-87.5) |
NSCLC: 60 Gy10 (54-87.5), SCLC: 43.1 Gy10
|
31.4 (16.9-45.9) |
-/76/64 |
12.9 (8.9-27.9) |
-/57/34 |
G1-2 Pneumonitis 19% |
|
G1-2 Esophagitis 19% |
|
Current study |
31 |
17.4 (4.8-76.8) |
15.1 (4.4-56.3) |
60 (45-66) |
50 (35-65) |
20.4 (4.8-76.8) |
96.8/76.8/39.4 |
15.4 (3.4-76.8) |
87.1/58.1/36.2 |
G1-2 Pulmonary 42% |
|
G1-2 Esophagitis 6.4% |

The median LRFS was also comparable with previous studies. In this study, the median LRFS was 15.4 months, while other studies showed an LRFS of 12.1-18 months [
11,
13,
14,
18]. Our definition of loco-regional recurrence was recurrence in the primary tumor and regional lymph node area. Since each study defines a loco-regional recurrence differently, such as recurrence in the high-dose irradiated volume only or in the ipsilateral lung and regional nodal area, it might be difficult to directly compare the LRFS value with those of other studies. In this study, sixteen patients showed progression or recurrence only in the high-dose irradiated volume, and only two patients showed progression in the regional lymph node. One patient had re-RT on the right paratracheal node, and then, 4.8 months later, there was progression around the descending aorta and the paraesophageal lymph node. Another patient had re-RT on the subcarinal lymph node only, and 23.1 months later, there was progression on the paratracheal station. Excluding these two patients to determine the median time to progression in the high-dose irradiated field only, which is defined as LPFS, our median LPFS was 15.4 months (range, 3.5 to 76.8 months).
The cumulative and re-RT BED
10 doses were significant factors for predicting a longer LRFS, and a high fraction size RT was marginally associated with a longer LRFS. According to De Bari et al. [
20], the LC rates with stereotactic re-RT were superior to those with conventional re-RT. Sumita et al. [
18] also implied that a re-RT dose ≥ 60 Gy was a prognostic factor for longer LPFS.
Furthermore, chemotherapy after re-RT, male sex, PTV at re-RT < 51.3 mL, GTV at re-RT < 10 mL and a fraction size > 3 Gy were significantly associated with longer DMFS, and a total BED10 dose ≥ 145 Gy10, and re-RT BED10 dose ≥ 68.7 Gy10 were associated with a marginally longer DMFS. A larger fraction size and a higher re-RT dose were predictive factors for longer LRFS, and these aggressive radiation factors improved the DMFS in recurrent lung cancer. A PTV at re-RT < 51.3 mL and, a GTV re-RT < 10 mL were significant factors for longer DMFS, which indicates that small volume tumors have a smaller cancer burden and should lead to longer DMFS. From this analysis, dose escalation should be considered in further studies to improve the outcome of re-RT treatment of small volume tumors.
Radiation-induced toxicities after re-RT remain a concern. In a pooled analysis of 14 studies, De Ruysscher et al. [
8] reported an average of 7% of patients exhibiting grade 3-4 lung toxic effects and 2% grade 3 esophagitis. Grade 5 bleeding was also reported in 12 of 408 patients.
Despite concerns about radiation-induced toxicities, there were no serious acute complications. Overall, the most frequent toxicities were pulmonary toxicities, including dyspnea, cough and pneumonitis (22.6% acute grade 1, 19.4% acute grade 2, 35.5% chronic grade 1, and 12.9% chronic grade 2). These results showed better rates than those reported after conventional RT. Compared with previous studies, our results indicated similar toxicity rates (
Table 6). Grade 3 pericarditis occurred in only one patient who also went through CAG with PCI due to underlying cardiac disease. This low rate of acute toxicity is probably due to the smaller RT target volumes and the high precision techniques commonly used for a course of salvage re-RT.
There were two patients of bleeding in locally progressive disease in re-RT volume. Both events were clinically diagnosed as hematemesis or hemoptysis; however, it is hard to determine the cause of the bleeding because they refused to be evaluated. Furthermore, both had taken anti-coagulants due to underlying disease. Based on clinical courses and images, it can be assumed that the causes of the bleeding are progressions of the local disease. As shown in
Figs. 3 and
4, two patients had an overlapped PTV in the central and higher cumulative D max dose of esophagus and trachea. (
Figs. 3 and
4).
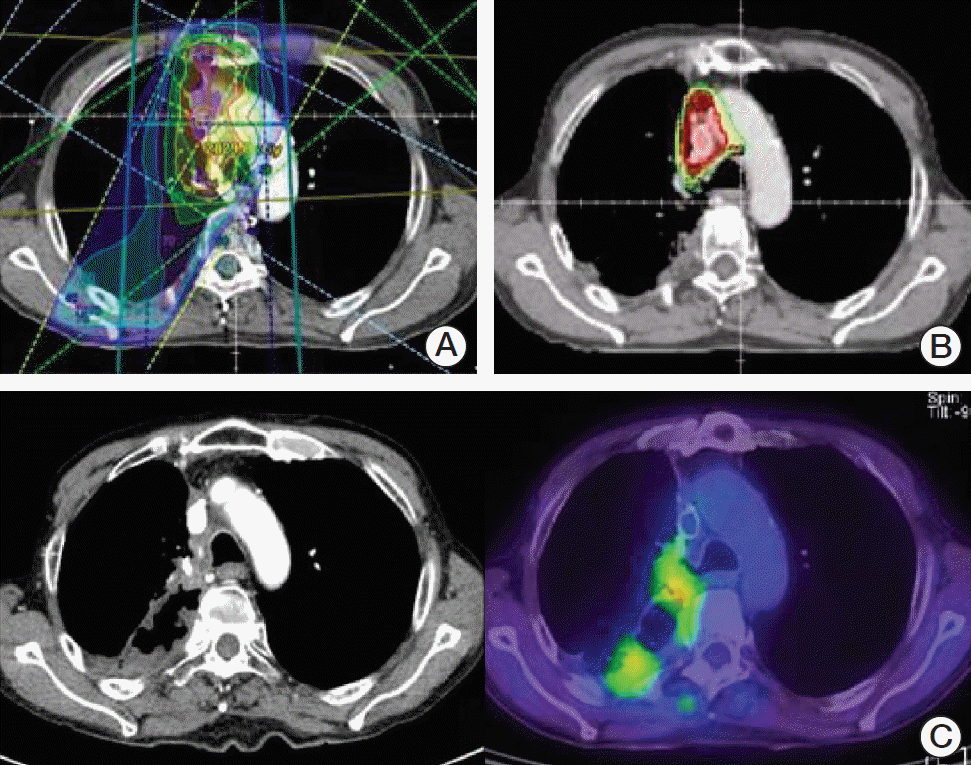 | Fig. 3.The initial irradiation (RT) plan (A), re-RT plan (B), and disease progression after re-RT (C) for patient 1 who experienced bleeding in locally progressive disease after high dose re-RT. 
|
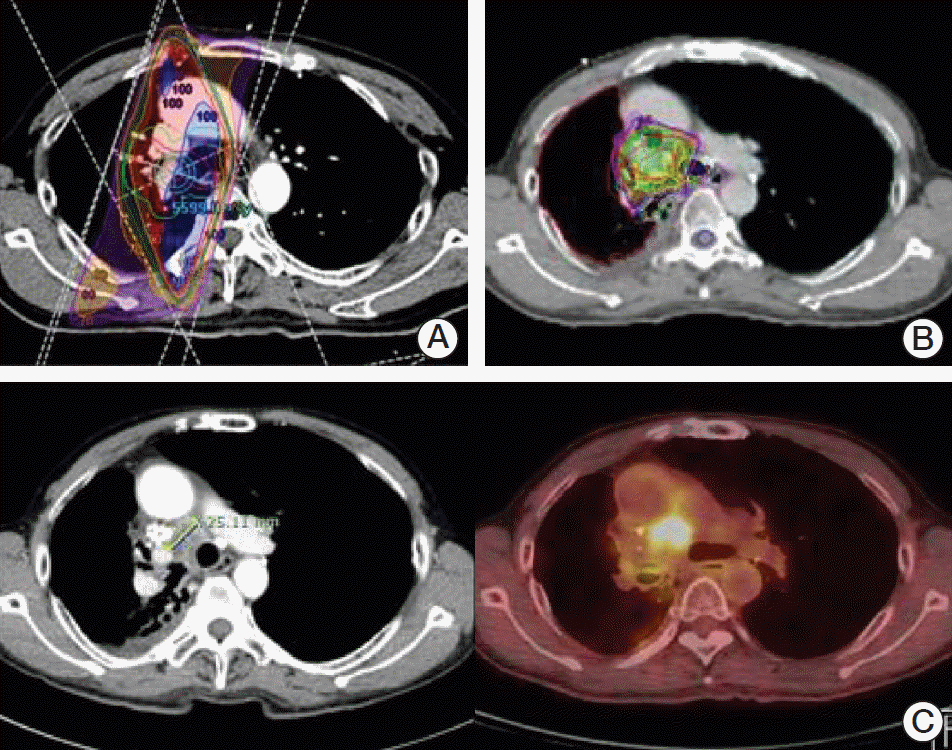 | Fig. 4.The initial irradiation (RT) plan (A), re-RT plan (B), and disease progression after re-RT (C) for patient 2 who experienced bleeding in locally progressive disease after high dose re-RT. 
|
The predictive factors for toxicity after re-RT have not been well-studied. Trovo et al. [
21], Peulen et al. [
22], and McAvoy et al. [
15] reported that toxic effects are greater in centrally located tumors. In this study, there were no significant differences in the location of tumors. The patients in this study had small PTV values, which might have led to less damage to the normal organ in which they were located. McAvoy et al. [
15] also reported that a CCRT effect may increase toxicity risk. The CCRT effect also did not show differences in this study. However, in this study, as the cumulative MLD increased, the acute pulmonary toxicity increased in a statistically significant manner.
This study has several limitations. First, the number of patients is small and the follow up period was not sufficiently long. This makes statistical analysis less accurate and may result in significant factors being missed. Because of the small number of patients, multivariate analysis was hard to be done. Statistical values with the small number of patients must be interpreted with cautions. Second, the patient’ characteristics and treatment techniques were not homogeneous, containing both SBRT and IMRT, and were also not homogeneous regarding pathology, stage, and treatment course. We tried to limit the heterogeneity by selecting patients who were diagnosed as non-metastatic or who had a controlledmetastatic stage for re-RT with high-dose salvage radiation. Third, owing to the retrospective methodology, the high-grade toxicity effects might be underestimated and there might be selection-bias.
Thoracic re-RT of lung cancer has been challenged by the tolerance doses of normal tissues, such as the lung, esophagus, and trachea and there is a high possibility of acute and chronic toxicities. However, our results showed a favorable OS rate with a low rate of toxicity.
The cumulative dose and re-RT dose were significant factors in predicting both longer OS and LRFS in this study.
To improve the treatment outcome and apply a sufficiently high dose, selection of patients to receive thoracic re-RT is crucial in extending the indications of re-RT. With proper indications and application of re-RT using highly precise technique, such as IMRT and SBRT, we are able to achieve improvements in treatment outcomes with acceptable toxicity in recurrent lung cancer.
Go to :





 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download