Introduction
Intense
Helicobacter pylori infection promotes a continuum of various macroscopic and histological changes in the gastric mucosa, including severe atrophic gastritis, intestinal metaplasia, dysplasia, and gastric cancer [
1-
4]. Intestinal metaplasia and dysplasia are considered premalignant lesions [
2]. However, approximately half of gastric cancer patients do not show intestinal metaplasia or dysplasia in background gastric mucosa [
5]. Even adjacent pairs of dysplasia and cancer tissues fail to display same mutations [
6]. This raises a question over whether
H. pylori infection induces dysplastic and neoplastic transformation of epithelial cells individually or sequentially. Intense
H. pylori infection that can elevate the risk of gastric cancer tends to disappear when progression of gastric mucosal atrophy with age creates poor tissue environment for bacterial colonization [
1,
3]. Therefore, a surrogate marker is required to identify an individual who has previously experienced
H. pylori infection with cancer risk.
Glandular stem cells maintaining gastrointestinal mucosa are periodically replaced with new stem cells. For example, an old glandular stem cell in each colonic gland is replaced by a new one every eight years [
7]. Pluripotent mesenchymal cells derived from various organ sites including adipose and bone marrow tissues can become new stem cells when native gastric stem cells are damaged by inflammation or when they lose their regenerative capacity [
8]. Newly adapted stem cells are capable of restoring undifferentiated histology and may be transformed into gastric cancer stem cells. Because mice rarely develop invasive gastric cancer [
9], few mouse models have confirmed the neoplastic transformation of marrow-derived stem cells [
10]. Humans and mice have distinct epigenetic structures around housekeeping genes. Epigenetic traits of housekeeping genes in humans can promote stem cell replacement but predispose stem cells to reactivating intrinsic migration traits [
11]. Undifferentiated-type gastric cancer is common in relatively younger individuals, compared to differentiated-type cancer [
12]. This suggests that new stem cells at an early stage of adaptation can preserve marrow properties. They are prone to transform into diffuse-type gastric cancer. Meanwhile, new stem cells at a late stage of adaptation are able to trans-differentiate into intestinal-type gastric cancer when they acquire gastric stem cell properties [
13]. It is likely that the development of gastric cancer is associated with phenotypic stability of periodically replaced stem cells.
In
H. pylori-infected stomach, numerous housekeeping genes containing CpG islands are concurrently methylated in an age-related manner [
14,
15]. It has been thought that concurrent CpG-island methylation can stabilize newly established phenotypes of stem cells [
11,
16,
17]. Such agerelated changes in CpG-island methylation will reach a peak during new stem cell replacement [
14]. Gastric mucosal atrophy spreading from the antrum to the body appears to impede phenotype-stabilizing methylation largely in the antrum [
15]. This can result in insufficient methylation in the cancer-prone antrum [
15,
18], thus raising the epigenetic risk of gastric cancer in association with mucosal atrophy. Meanwhile, the gastric body with a minimal mucosal atrophic effect exhibits frequent CpG-island overmethylation in patients with cancer, indicating that a large number of methylation-inducible new stem cells are present over the background mucosa [
15]. Therefore, cancer-related period of stem cell replacement can be estimated by analyzing the period of CpG-island overmethylation and the level of gastric mucosal atrophy.
There are transitional-CpG sites on methylation gradients between weakly methylated transcriptional start sites (TSS) and densely methylated retroelements [
16,
17,
19,
20]. These transitional-CpG sites display age-related methylation changes in the
H. pylori–infected stomach, providing useful epigenetic information about the period of stem cell replacement [
14,
15]. The window period between
H. pylori infection and cancer appearance, during which
H. pylori infection disappeared with the progression of gastric mucosal atrophy, was investigated based on the age of transitional-CpG overmethylation.
Discussion
H. pylori infection induces a various number of methylation-inducible mucosal cells from individual to individual. Despite the cyclic replacement of glandular stem cells [
7], few studies have demonstrated cyclic methylation peaks.
TFF2 is strongly expressed in both the antrum and body, playing a master role in induction of stem cell trans-differentiation [
14,
17]. Strong expression of this master gene made no increase in
H. pylori–associated methylation. Antrum-specific
TFF3 and body-specific
GHRL were undermethylated in the antrum and body, respectively [
14]. This is in accordance with an inverse relationship between transitional-CpG methylation and gene expression [
23]. Strong expression of master genes is organized by priority to reestablish new stem cell phenotypes and to lead stem cell repopulation [
17]. The tissue-specific methylation can periodically fluctuate under the influence of gene expression after the establishment of new stem cell phenotypes [
24]. The results of stomach-specific gene methylation might represent the cyclic repopulation of stem cells under the influence of gene expression rather than by accidental events.
Housekeeping genes containing CpG islands made slow methylation curves. This suggests that CpG-island genes keep their overmethylation for a long time in order to stabilize newly established cell phenotypes.
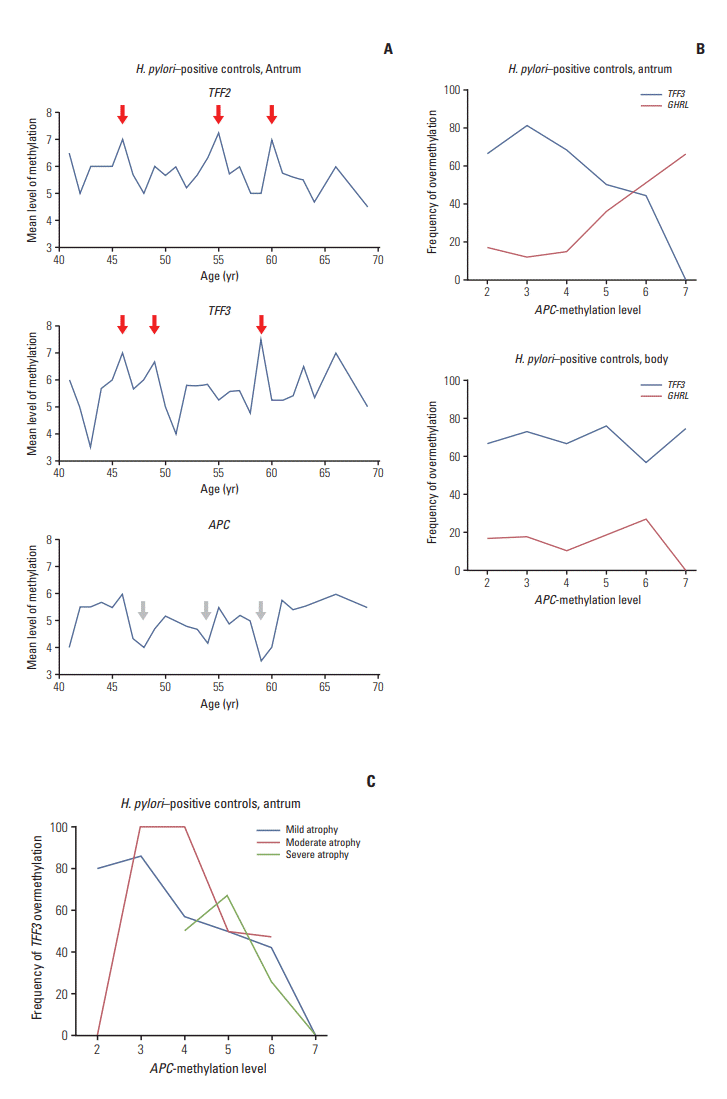
H. pylori–associated
TFF3 methylation was found to mark three overmethylation peaks at three levels of gastric mucosal atrophy (
Fig. 2C). Rapid
TFF3 overmethylation was found earlier than
Alualone overmethylation in
H. pylori–positive controls with mild atrophy, indicating an early stage of overmethylation. The age of
TFF3+
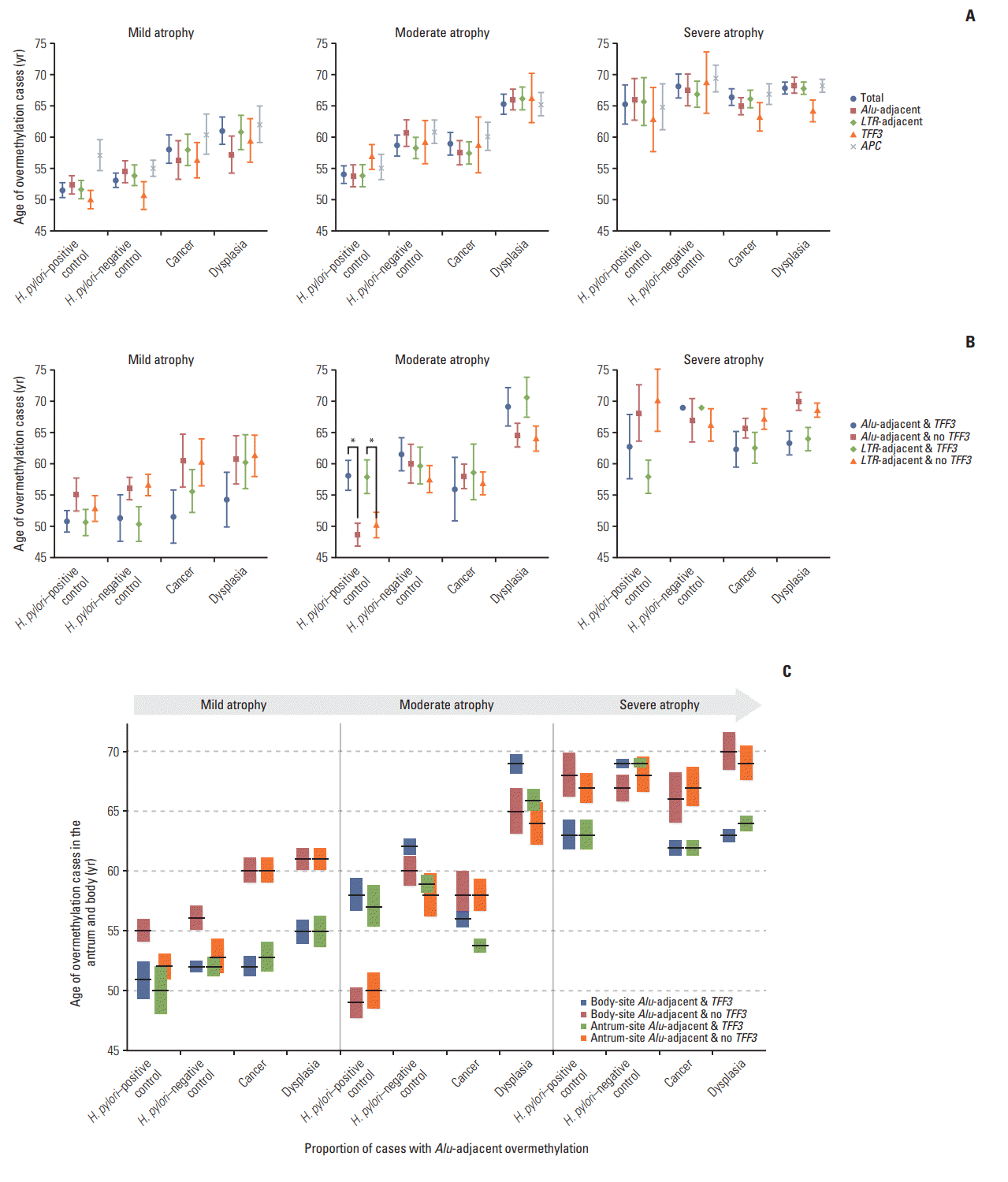
Alu overmethylation appeared at similar intervals among mild (51 years), moderate (58 years), and severe (63 years) atrophic cases. Whereas, the age of moderate atrophic cases (54 years) was close to that of mild atrophic cases (52 years). The age of stomach-specific gene methylation is thought to represent cyclic methylation changes at certain times.
H. pylori–positive controls with moderate mucosal atrophy demonstrated
TFF3 overmethylation existing in a wide range of
APC methylation (
Fig. 2C). This implies that the moderate atrophic cases involve several methylation cycles. There was a significant difference in the mean age between early
Alualone overmethylation and late
TFF3+
Alu overmethylation in the moderate mucosal atrophic cases (
Fig. 3B).
H. pylori–positive controls with moderate mucosal atrophy appeared to include two distinct cycles of transitional-CpG methylation, each of which resulted from early-onset and late-onset mucosal atrophy. Early-onset moderate mucosal atrophy is likely to impede
TFF3 overmethylation at primary methylation cycle. Meanwhile, late
TFF3+
Alu overmethylation at the mean age of 58 years presents early overmethylation in cases of late-onset mucosal atrophy. The age of early
Alu-alone overmethylation (49 years) in moderate atrophic cases was comparable with that of
TFF3+
Alu overmethylation in mild atrophic cases (51 years). This suggests that the cyclic repopulation of stem cells is initiated at a certain period regardless of the level of gastric mucosal atrophy.
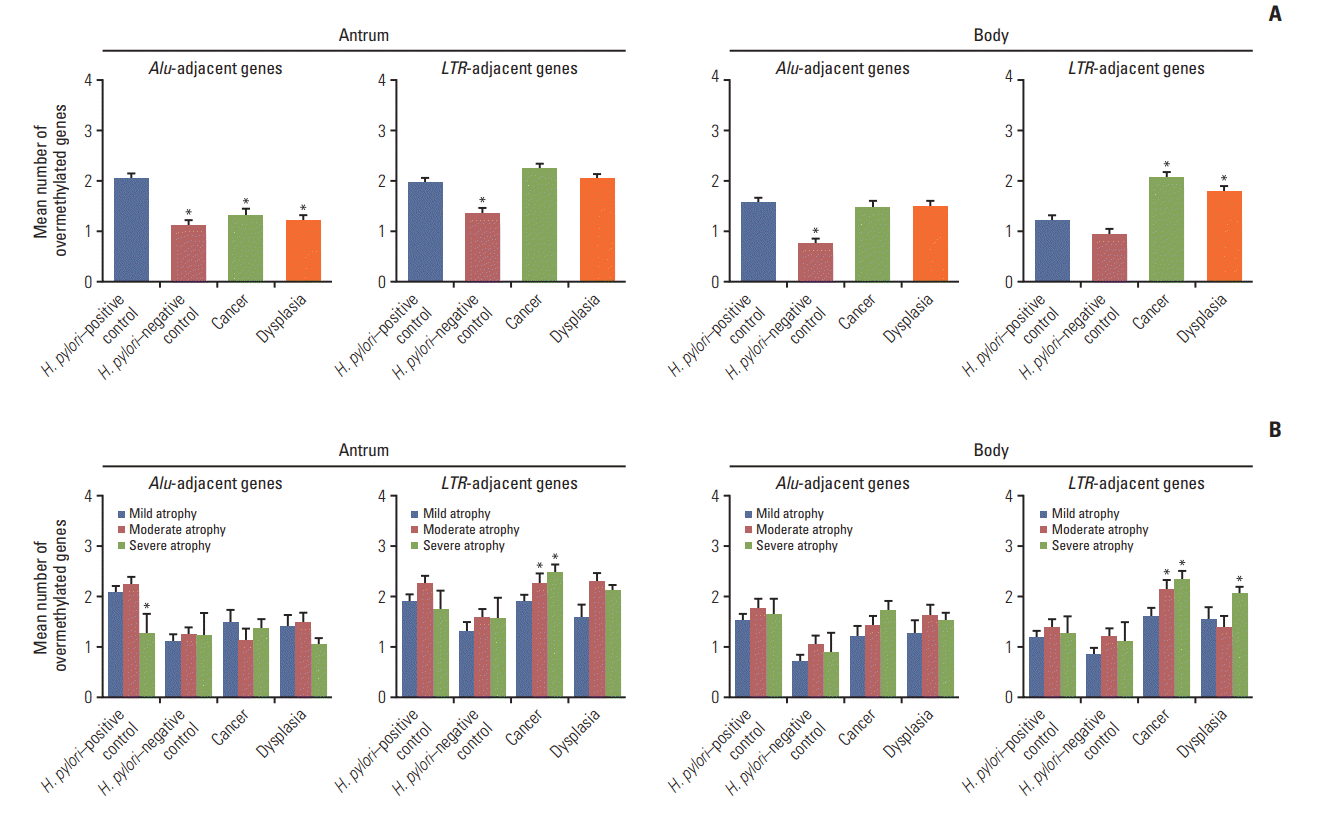
LTR-adjacent overmethylation not
Alu-adjacent overmethylation was significantly frequent in cancer patients (
Fig. 1). In cancer patients, early-onset atrophic changes, inducing a high number of methylation-inducible cells, might fail to increase
Alu-adjacent methylation. Indeed, previous studies on
Alu retroelements have demonstrated hypomethylation changes in
H. pylori-infected subject with severe atrophy and patients with gastric cancer [
25].
Alu-adjacent overmethylation was found to be later in gastric cancer patients with moderate atrophy (58 years) than in
H. pylori–positive controls with mild (51 years) or moderate atrophy (49 years) (
Fig. 3B). The
Alu-adjacent CpG-island genes can produce
Alu RNAs to quickly stabilize cell phenotypes prior to transitional-CpG methylation [
11,
17]. There appears to be a window of
Alu RNA-dependent stabilization during the expansion of new stem cells. Cancer-risk moderate atrophy may prolong the
Alu RNA-dependent step of phenotypic stabilization for a cycle of glandular cell repopulation. The risk of gastric cancer seems to be initiated with impeding phenotype-stabilizing methylation of new stem cells.
LTR-adjacent overmethylation was frequent in both cancer and dysplasia patients at a severe level of gastric mucosal atrophy (
Fig. 1B). Methylation-inducible cells are likely to transform into cancerous cells and subsequently into dysplastic cells.
The proportion of
TFF3+
Alu overmethylation was low in gastric cancer (20%) and dysplasia (17%) patients with severe atrophic cases (
Fig. 3C). However, the proportion of
Aluadjacent overmethylation tended to be increased in both cancer and dysplasia patients (
Fig. 3C). Such increased methylation of CpG-island genes can change the epigenetic status of new stem cells from unstable to stable during subsequent cell repopulation. Gastric mucosa appears to be at a relatively low risk for cancer in severe atrophic cases, even though neoplastic transformation into both gastric cancer and dysplasia occur frequently in such cases.
CpG-island methylation was not increased in dysplasia patients with moderate atrophy (66 years) who were older than gastric cancer patients (58 years) (
Figs. 1B and
3A). This suggests that weak
H. pylori infection introduces late-onset slow-progressing gastric mucosal atrophy, staying at a moderate level, and leads to a low number of methylationinducible cells.
In previous prospective studies, intense early-onset
H. pylori infection has been found to be able to elevate the risk of gastric cancer, which will induce severe mucosal atrophy eventually [
1,
3]. Both cancer (66 years) and dysplasia (68 years) patients with severe atrophy showed frequent LTRadjacent overmethylation at similar ages (
Figs. 1B and
3A). It is likely that early-onset moderate atrophy progresses rapidly toward severe atrophy. The window period for developing gastric cancer is predictable based on the following sequence of
H. pylori-associated events: intense
H. pylori infectionearly-onset rapid-progressing moderate atrophy impeded
Alu-adjacent methylation and increased LTRadjacent methylationsevere atrophy.
Cancer risk of gastric mucosal atrophy has been assessed previously by endoscopic recognition of the atrophic border and by the Sydney system including histologic examination of biopsies [
21,
26]. There are good agreements between the two atrophy-staging systems. A prospective study on gastric cancer occurrence, involving 5,373 subjects (mean age, 50 years) over 10 years has reported that the relative risk of gastric cancer is the greatest among subjects with moderate atrophy [
27]. The peak value of relative risk is reached after 4-6 years of follow-up, followed by attenuation with age and severe atrophic changes. These findings are in accordance with our results showing that rapid-progressing atrophy impeding methylation (mean age, 58 years) is followed by severe atrophy with a low risk of gastric cancer. However, atrophy-staging systems provide little practical information at the time of endoscopic examination because neither endoscopic findings nor histological parameters are available for the identification of rapid-progressing moderate atrophy. On the other hand, cancer-related methylation type associated with an increased risk of gastric cancer can be useful for highrisk individual detection at a moderate level of gastric mucosal atrophy (
Table 2).
Previous studies on
H. pylori-associated methylation have mainly concentrated on weakly methylated CpG-island centers without considering transitional-CpG sites [
18]. There are considerable inter-observer differences on the association of hypermethylated CpG-island centers with inactivation of tumor suppressor genes (
S9 Table) [
28]. When the type of retroelements adjacent to genes examined in previous studies was analyzed, most CpG islands were classified as
Alu retroelements. Only a small number of CpG islands were classified as
LTR retroelements or no retroelement. Among six studies on the CpG-island centers of
LTR-adjacent CDKN2A, two displayed a higher level of methylation in cancer patients than that in
H. pylori-positive controls. However, this was not observed in the other four studies. These inconsistent results might be due to weak methylation of the CpGisland centers that show a lack of reproducibility with different detection methods and various arbitrary cutoff points. The transitional-CpG sites displayed a wide range of methylation variations compared with the CpG-island centers in cell-differentiation and human gastric cancer studies [
19,
20]. In addition, site-specific methylation changes were clearly observed in the transitional-CpG sites, but not in the CpGisland centers [
15,
18]. Therefore, the transitional-CpG sites are profitable for clarifying
H. pylori-associated methylation (
Fig. 1A).
A phenotypic shift from undifferentiated to differentiated types is clearly observed in both intestinal metaplasia and gastric cancer [
12,
13]. These findings imply that new gastric stem cells at an early adaptation stage are prone to restore properties of mesenchymal cells. So the possibility of metaplastic or neoplastic differentiation reaches a peak at an unstable step of phenotypic stabilization, suggesting that both intestinal metaplasia and gastric cancer appear as a paracancerous phenomenon, not a precancerous cascade [
29]. Gastric dysplasia may also result from a paracancerous phenomenon. Gastric cancer is closely associated with highgrade dysplasia compared with low-grade dysplasia [
30]. Morphological similarities of gastric dysplasia and cancer implicate a cause-and-effect relationship between gradual phenotypic stabilization and aberrant differentiation rather than a dysplasia-cancer sequence. Accordingly, gastric dysplasia might occur earlier or later than gastric cancer for the period of impeded CpG-island methylation.
In conclusion, moderate atrophy of the gastric mucosa impedes phenotype-stabilizing Alu-adjacent methylation for nine years (a cycle of glandular stem cell repopulation) in gastric cancer patients, but not in dysplasia patients. The risk of gastric dysplasia could arise later than that of gastric cancer during phenotypic stabilization which grows with transitional-CpG methylation. The window period for developing gastric cancer may be predicted by analyzing the age of transitional-CpG methylation and the level of gastric mucosal atrophy.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download