Abstract
Purpose
Determine the frequency and prognostic value of circulating Epstein-Barr virus (EBV) DNA copy number in angioimmunoblastic T-cell lymphoma (AITL) patients who were treated with dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin (DA-EPOCH) regimens.
Materials and Methods
Sixty newly-diagnosed AITL patients were retrospectively enrolled in the present study. All patients were treated with DA-EPOCH regimen.
Results
Twenty-two subjects (36.7%) had a EBV DNA-positive test at diagnosis. EBV DNA‒positive patients were associated with lower lymphocyte-monocyte ratio (p=0.024). Median follow-up was 40 months (range, 14 to 100 months). The overall response rate for all the 60 AITL patents were 71.7% (95% confidence interval [CI], 58.6 to 82.5) with 3-year progressive-free survival (PFS) rate of 30.9%±6.1% and overall survival (OS) rate of 60.1%±6.6%. Not only did PFS estimation differ between the EBV DNA‒positive and EBV DNA‒negative group (hazard ratio [HR], 2.24; 95% CI, 1.15 to 4.35; p=0.006), but also worse OS was observed in the pretreatment EBV DNA‒positive group than in the EBV DNA‒negative group (HR, 2.74; 95% CI, 1.22 to 6.19; p=0.006). EBV DNA test positivity was independent prognostic marker for both PFS (HR, 2.17; 95% CI, 1.17 to 4.00; p=0.014) and OS (HR, 3.24; 95% CI, 1.48 to 7.11; p=0.004) after adjusting International Prognostic Index and prognostic index for AITL score. Reduction in EBV copies was significantly associated with therapy-response.
Angioimmunoblastic T-cell lymphoma (AITL) is one of the most frequent peripheral T-cell lymphomas and clinically characterized by widespread lymphadenopathy, and commonly, extranodal disease, immune-mediated hemolysis, and polyclonal hypergammaglobulinemia [1]. Both the neoplastic T cells of T follicular helper (TFH) cells and tumor microenvironment (TEM) which contained reactive component made of small lymphocytes, histiocytes, eosinophils, plasma cells, and B immunoblasts are involved in the maintenance of tumor cell viability [2,3].
Within this AITL TEM, some of the B immnunoblasts are Epstein-Barr virus (EBV)‒positive, which can drive the development of a EBV-positive B-cell lymphoma [4]. However, the pathogenetic role of EBV infection/reactivation of these B cells in AITL development is unclear. It is unknown whether EBV-positive B cells are involved in AITL lymphomagenesis and development of the TEM or merely occur as a sequence of an underlying “immune dysfunction” [4-6]. The prognostic roles of EBV DNA in plasma or whole blood have been explored in many other EBV-associated lymphomas [7-12]. However, no studies were specially focused on the prognostic role of EBV DNA in whole blood for AITL patients. In this study, we focused on the frequency and prognostic roles of EBV DNA in 60 AITL patients who were all treated with the regimen of dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin (DA-EPOCH) [13,14].
Sixty consecutive newly-diagnosed AITL patients were enrolled in this study from February, 2009 to November, 2015. Diagnosis of AITL was based on criteria of 2008 World Health Organization classification [1]. Baseline clinical characteristics were totally available, including age, sex, Ann Arbor stage, B symptoms, Eastern Cooperative Oncology Group (ECOG) performance status, extranodal sites, lactate dehydrogenase (LDH), platelet counts (PLT), lymphocytemonocyte ratio (LMR), bone marrow involvement (BMI), prognostic index for angioimmunoblastic T-cell lymphoma (PIAI) score (which including age > 60, ECOG performance status > 2, extranodal sites of disease > 1, presence of B symptoms, and a PLT < 150×109/L). Furthermore, anti-EBV antibody tests (Epstein-Barr nuclear antigen [EBNA]-IgG, EBV-CA-IgG, EBV-CA-IgA, EBV-CA-IgM, and EBV-EA-IgG) were done in some subjects. All patients were treated with DA-EPOCH regimen [13,14]. Response criteria were reported using standard criteria [15] and response were classified as complete response (CR), unconfirmed CR (CRu), partial response (PR), stable disease, and progressive disease (PD).
Whole blood samples were collected in an EDTA-containing tube and DNA extracted with the EBV-PCR Fluorescence Quantitative Diagnostic Kit (Da An Gene Co., Guangzhou, China). Quantification of EBV-specific sequences was performed by RQ-PCR assay with an ABI PRISM 7500 (Applied Biosystems, Foster City, CA). Copy number was calculated from a standard curve. The lower boundary of test sensitivity was 5×103 copies/mL. Subjects with values < 5×103 copies/mL were scored as EBV-DNA-negative including potentially subjects with no copies and subjects with values of 1 to < 5×103 copies/mL.
Fisher exact test was applied to categorical variables and the Mann-Whitney U test to continuous variables. The discriminative ability of the characteristics was determined using time-dependent receiver-operator characteristics curves and the corresponding areas under the curve (AUCs) were calculated to assess the predictive accuracy of the characteristics. Progressive-free survival (PFS) was defined as interval from diagnosis to first progression, relapse. Overall survival (OS) was defined as interval from diagnosis to death, loss to follow-up or July, 2017. Survival curves were constructed by Kaplan-Meier method, and log-rank test used to test for significant differences. Associations between variables and EBV DNA testing results were interrogated in univariable analyses. Variables with significant associations were included in multivariable Cox proportional hazards regression analyses. Statistical analyses were performed using SPSS software for Windows ver. 17.0 (SPSS Inc., Chicago, IL). p-values < 0.05 were considered significant. Data were analyzed as of July 1, 2017. Median follow-up is 40 months (range, 14 to 100 months).
Twenty-two subjects (36.7%) had a positive EBV DNA test at diagnosis. Median viral concentration was 7.20×105 copies/mL (range, 8.36×103 to 5.58×106 copies/mL). Only 10 patients had pretreatment results of anti-EBV antibody tests. And all the 10 subjects had serological evidence of prior EBV-infection (EBNA-IgG–positive and EBV-CA-IgG–positive). Three out of the 10 subjects also had EBV-EA-IgG–positive and all the patients were both EBV-CA-IgA–negative and EBV-CA-IgM–negative. Clinical variables are compared between the EBV DNA–positive and –negative cohorts in Table 1. The median age was 60 years (range, 33 to 79 years) and 43.3% of them were more than 60 years old. There was a male predominance (38 men and 22 women). Most patients (88.3%) were at advanced stage and 58.3% had elevated LDH levels. B symptoms were observed in 66.7%. Twenty-one patients (35.0%) had an ECOG performance status (PS) score of more than 2, and 23 patients (38.3%) had more than one extranodal sites. BMI was found in 17 patients (28.3%) and 45 patients (75.0%) had PIAI score of 3-5. The optimal cut-off value for defining LMR according to OS was 1.7 (sensitivity, 45%; specificity, 91%; AUC, 0.668; p=0.024) and this cut-off point also had significant predictive value for PFS. Among the 21 patients who had LMR less than 1.7, there were 12 patients in EBV DNA‒positive group while nine patients in EBV DNA‒negative group (p=0.024). However, no significant differences were observed between EBV DNA–positive and –negative group according to other clinical characteristics (Table 1).
Response at the end of the treatment was assessed after treatment completion for 60 patients. Seven patients received upfront autologous hematopoietic stem cell transplantation (ASCT) after completion of six cycles of DA-EPOCH while only one patient received ASCT after salvage chemotherapy. And one patient received allogeneic hematopoietic stem cell transplantation after salvage chemotherapy. Twenty-two patients achieved a CR or CRu (36.7%; 95% confidence interval [CI], 24.6 to 50.1). Twenty-one patients (35.0%; 95% CI, 23.1 to 48.4) had a partial response, leading to an overall response rate (ORR) of 71.7% (95% CI, 58.6 to 82.5). With a median follow-up of 40 months (range, 14 to 100 months), the 3-year PFS rate was 30.9%±6.1% and the OS rate was 60.1%±6.6% (Fig. 1A). Twenty-seven patients (45.0%) were dead. Among these patients, 22 patients (81.5%) were attributed to disease progression and the other five patients (18.5%) were to serious infection. Of note, no patients developed diffuse large B-cell lymphoma during the follow-up period. For EBV DNA–positive patients (22 patients), the 3-year PFS rate was 6.8%±6.1% and the OS rate was 43.8%±11.0% while for EBV DNA‒negative group (38 patients), 3-year PFS rate was 44.7%±8.1% and the OS rate was 69.4%±7.9%.
Not only did PFS estimation differ between the EBV DNA–positive and EBV DNA–negative group (HR, 2.24; 95% CI, 1.15 to 4.35; p=0.006), but also worse OS was observed in the pretreatment EBV DNA–positive group than in the EBV DNA–negative group (HR, 2.74; 95% CI, 1.22 to 6.19; p=0.006); this is shown in Fig. 1B and C.
Variables significantly associated with PFS in univariable analyses were entered into multivariate analyses. BMI involvement (HR, 2.26; 95% CI, 1.17 to 4.35; p=0.015), PLT < 150×109/L (HR, 2.52; 95% CI, 1.34 to 4.74; p=0.004) and EBV-DNA positivity (HR, 2.17; 95% CI, 1.17 to 4.00; p=0.014) were significantly associated with PFS (Table 2). Variables significantly associated with OS in univariable analyses were entered into multivariable analyses. ECOG score of 2-4 (HR, 2.61; 95% CI, 1.21 to 5.64; p=0.015), LDH > 270 U/L (HR, 2.60; 95% CI, 1.08 to 6.27; p=0.034) and EBV DNA positivity (HR, 3.24; 95% CI, 1.48 to 7.11; p=0.004) were significantly associated with OS (Table 2).
Twenty-two subjects with an EBV DNA‒positive test at diagnosis had sequential measurements of EBV copies during therapy and follow-up (Fig. 2). Eight patients who experienced PD before the completion of six cycles of DA-EPOCH were all with elevated EBV DNA copy number. And six of eight patients were dead at the end of follow-up deadline. Among the seven patients achieved PR at the end of completion of DA-POCH treatment, only three patients were with undetectable EBV-DNA copy number and two of these three patients were alive at the end of follow-up deadline. As regard to the seven patients who were assessed as CR/CRu at the end of completion of DA-EPOCH treatment, the copy number of EBV-DNA was all decreased into undetectable level while five of them experienced PD during the follow-up.
Similar to natural killer/T-cell lymphoma, AITL has been showed to have an intricate relationship to EBV. The majority of large B cells in the TEM can be shown by in situ hybridization to have active EBV while the malignant TFH will not [4-6]. However, the nature of the relationship between EBV and AITL is unclear. Some argue that the presence of EBV reflects the profound immune-deficient state for AITL patients which are also demonstrated in the present study with the fact that more EBV DNA‒positive patients were associated lower LMR [2-4]. However, others argue that EBV itself drives the development of AITL [2-4,16]. Delfau-Larue et al. [17] demonstrated that EBV detection in peripheral blood mononuclear cell correlated strongly with EBV quantification by Epstein-Barr encoding region in lymph node biopsies and the presence of circulating EBV DNA is strongly associated with the presence of circulating AITL tumor cells. Recently, Chen et al. [12] showed that elevated plasma EBV DNA was associated with shorter survival in 135 peripheral T-cell lymphoma patients which included 25 AITL patients. Here, we focused on the entity of AITL subtype which were also based on the same regimen of DA-EPOCH, and found that EBV-DNA in whole blood were independent risk factor of survivals (PFS and OS) for 60 AITL patients and reduction in EBV copies was significantly correlated with therapy-response.
Overall, the prognosis for AITL patients is poor with the median OS of less than 3 years in most studies [6]. Many treatment strategies, ranging from intensive combination chemotherapy to adding new drug to conventional chemotherapy have been evaluated for AITL patients. Adding new drugs of etoposide and bleomycin in the MACOP-B to CHOP regimen have failed to increase the survival rate for AITL patients [18,19]. Given the important role of TEM in AITL patients, some attempts to add the drug of attacking the vascular irregularities and B-cell blast to CHOP have been evaluated in AITL patients [17,20,21]. Avastin, a vascular endothelial growth factor antagonist has been combined with CHOP with avastin maintenance in a phase II study. However, due to the significant cardiac toxicity, it was not further explored [20,21]. Rituximab, targeting B-cells was also employed in combination with CHOP in a phase II study, which included 25 AITL patients [17]. The ORR was 80% with 2-year OS rate of 62% which was close to that previously reported with CHOP alone [2]. In the present study, all patients received DA-EPOCH regimen. Overall, the ORR was 71.7% with the 3-year PFS rate of 30.9% and OS rate of 60.1%. It seemed that no significant improvements were observed for patients who were treated with more intensive chemotherapy of DA-EPOCH than the regimen of CHOP or R-CHOP [2,17,22], though it has been proposed in the NCCN guidelines based on institutional preference.
However, when specialized in the EBV-DNA subgroup, we found that patients with EBV-DNA negative have superior survivals with the ORR of 81.6% and 3-year PFS and OS rate of 45.0% and 70.0%, respectively. After deep analysis of the dynamic quantitative changes of EBV-DNA copy numbers with the therapy, we found that nearly all the patients with early disease progression who were experienced PD after 3 cycles of DA-EPOCH therapy were with elevated EBV-DNA copy number (subject 1, 2, 3, and 4). For patients who achieved PR after three cycles of DA-EPOCH while also were with elevated EBV DNA copy number, they all experienced PD before the completion of six cycles of DA-EPOCH regimen (subject 5, 6, 7, and 8). In our cohort of 22 EBV DNA–positive patients, only patients had undetectable EBV-DNA copy number after the one cycle of DA-EPOCH regimen and keep undetectable levels until the completions of all the 6-8 cycles of DA-EPOCH regimen have the best prognosis who were all alive with the CR/CRu (subject 21 and 22). Therefore, we concluded that: (1) similar to EBV associated hemophagocytic lymphohistiocytosis patients, early blocking of EBV storm was important for pretreatment EBV DNA‒positive AITL patients; (2) New therapy to anti-EBV was urgently needed for those patients to improve their survivals.
Due to the low prevalence of AITL, limit data were available on prognostic factors in patients with AITL. International Prognostic Index, as a classic model for risk stratification of patients with aggressive non-Hodgkin lymphoma, failed to estimate survivals in AITL patients [23]. PIAI which including age > 60 years, ECOG PS > 2, extranodal sites of disease > 1, presence of B symptoms, and a PLT < 150×109/L) first described by Federico et al. [24] were specifically for AITL patients with a low risk group (0-1) with a 5-year OS rate of 44% and high risk group (2-5) associated with 5-year OS rate of 24%. In our study, PIAI score of 2-5 was only independently associated with inferior OS while not PFS. Furthermore, we found that pretreatment EBV DNA positive was independent risk factor for both PFS and OS after adjusting PIAI. Recently, Kim et al. [8] has suggested that circulating EBV-DNA level should be added into the prognostic index for natural killer lymphoma (PINK-E), which were also validated in our previous study [11]. Therefore, we thought that EBV DNA copy number should also be added into the prognostic index such as PIAI or any other prognostic index which might be conducted with larger cohort of AITL patients in the further for better risk-stratification.
Though a number of weaknesses, such as retrospective study with relatively small number size, were in the present study, we still come up with some indications for AITL patients: (1) intensive chemotherapy with DA-EPOCH cannot significantly improve outcomes for AITL patients; novel therapies especially combined the anti-EBV therapy are urgently needed for those patients; (2) pretreatment EBV DNA copy number was an important prognostic and monitoring marker for AITL patients which should be added into the further prognostic index based on larger prospective studies specially for AITL patients.
ACKNOWLEDGMENTS
This study was supported by National Natural Science Foundation of China (81370657, 81470328, 81600130, 81770166, 8172010-8002), Jiangsu Province’s Medical Elite Programme (ZDRCA2016022), Project of National Key Clinical Specialty, National Science & Technology Pillar Program (2014BAI09B12), Jiangsu Provincial Special Program of Medical Science (BL2014086 and BE2017751) and National Science and Technology Major Project (2017ZX09304032).
References
1. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classication of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press;2008.
2. Mourad N, Mounier N, Briere J, Raffoux E, Delmer A, Feller A, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d'Etude des Lymphomes de l'Adulte (GELA) trials. Blood. 2008; 111:4463–70.

3. Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005; 5:853–65.

4. Zhou Y, Attygalle AD, Chuang SS, Diss T, Ye H, Liu H, et al. Angioimmunoblastic T-cell lymphoma: histological progression associates with EBV and HHV6B viral load. Br J Haematol. 2007; 138:44–53.

5. Huang J, Zhang PH, Gao YH, Qiu LG. Sequential development of diffuse large B-cell lymphoma in a patient with angioimmunoblastic T-cell lymphoma. Diagn Cytopathol. 2012; 40:346–51.

6. Hsu SM, Hsu PL. Autocrine and paracrine functions of cytokines in malignant lymphomas. Biomed Pharmacother. 1994; 48:433–44.

7. Ito Y, Kimura H, Maeda Y, Hashimoto C, Ishida F, Izutsu K, et al. Pretreatment EBV-DNA copy number is predictive of response and toxicities to SMILE chemotherapy for extranodal NK/T-cell lymphoma, nasal type. Clin Cancer Res. 2012; 18:4183–90.

8. Kim SJ, Yoon DH, Jaccard A, Chng WJ, Lim ST, Hong H, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol. 2016; 17:389–400.

9. Liang JH, Gao R, Xia Y, Gale RP, Chen RZ, Yang YQ, et al. Prognostic impact of Epstein-Barr virus (EBV)-DNA copy number at diagnosis in chronic lymphocytic leukemia. Oncotarget. 2016; 7:2135–42.

10. Liang JH, Lu TX, Tian T, Wang L, Fan L, Xu J, et al. Epstein-Barr virus (EBV) DNA in whole blood as a superior prognostic and monitoring factor than EBV-encoded small RNA in situ hybridization in diffuse large B-cell lymphoma. Clin Microbiol Infect. 2015; 21:596–602.

11. Liang JH, Wang L, Peter Gale R, Wu W, Xia Y, Fan L, et al. Efficacy of pegaspargase, etoposide, methotrexate and dexamethasone in newly diagnosed advanced-stage extra-nodal natural killer/T-cell lymphoma with the analysis of the prognosis of whole blood EBV-DNA. Blood Cancer J. 2017; 7:e608.

12. Chen Y, Zheng X, Chen B, Yang X, Zheng J, Zheng Z, et al. The clinical significance of Epstein-Barr virus DNA in peripheral blood mononuclear cells in patients with non-Hodgkin lymphoma. Leuk Lymphoma. 2017; 58:2349–55.

13. Lai GM, Chen YN, Mickley LA, Fojo AT, Bates SE. P-glycoprotein expression and schedule dependence of adriamycin cytotoxicity in human colon carcinoma cell lines. Int J Cancer. 1991; 49:696–703.

14. Wilson WH, Grossbard ML, Pittaluga S, Cole D, Pearson D, Drbohlav N, et al. Dose-adjusted EPOCH chemotherapy for untreated large B-cell lymphomas: a pharmacodynamic approach with high efficacy. Blood. 2002; 99:2685–93.

15. Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999; 17:1244.
16. Weiss LM, Jaffe ES, Liu XF, Chen YY, Shibata D, Medeiros LJ. Detection and localization of Epstein-Barr viral genomes in angioimmunoblastic lymphadenopathy and angioimmunoblastic lymphadenopathy-like lymphoma. Blood. 1992; 79:1789–95.

17. Delfau-Larue MH, de Leval L, Joly B, Plonquet A, Challine D, Parrens M, et al. Targeting intratumoral B cells with rituximab in addition to CHOP in angioimmunoblastic T-cell lymphoma: a clinicobiological study of the GELA. Haematologica. 2012; 97:1594–602.

18. Nickelsen M, Ziepert M, Zeynalova S, Glass B, Metzner B, Leithaeuser M, et al. High-dose CHOP plus etoposide (Mega-CHOEP) in T-cell lymphoma: a comparative analysis of patients treated within trials of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Ann Oncol. 2009; 20:1977–84.

19. Karakas T, Bergmann L, Stutte HJ, Jager E, Knuth A, Weidmann E, et al. Peripheral T-cell lymphomas respond well to vincristine, adriamycin, cyclophosphamide, prednisone and etoposide (VACPE) and have a similar outcome as high-grade B-cell lymphomas. Leuk Lymphoma. 1996; 24:121–9.

20. Ganjoo K, Hong F, Horning SJ, Gascoyne RD, Natkunam Y, Swinnen LJ, et al. Bevacizumab and cyclosphosphamide, doxorubicin, vincristine and prednisone in combination for patients with peripheral T-cell or natural killer cell neoplasms: an Eastern Cooperative Oncology Group study (E2404). Leuk Lymphoma. 2014; 55:768–72.

21. Advani RH, Hong F, Horning SJ, Kahl BS, Manola J, Swinnen LJ, et al. Cardiac toxicity associated with bevacizumab (Avastin) in combination with CHOP chemotherapy for peripheral T cell lymphoma in ECOG 2404 trial. Leuk Lymphoma. 2012; 53:718–20.

22. de Leval L, Gisselbrecht C, Gaulard P. Advances in the understanding and management of angioimmunoblastic T-cell lymphoma. Br J Haematol. 2010; 148:673–89.

23. International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993; 329:987–94.
Fig. 1.
(A-C) Progressive-free survival (PFS) (B) and overall survival (OS) (C) for 60 patients with the analysis of pretreatment Epstein-Barr virus (EBV) DNA status.
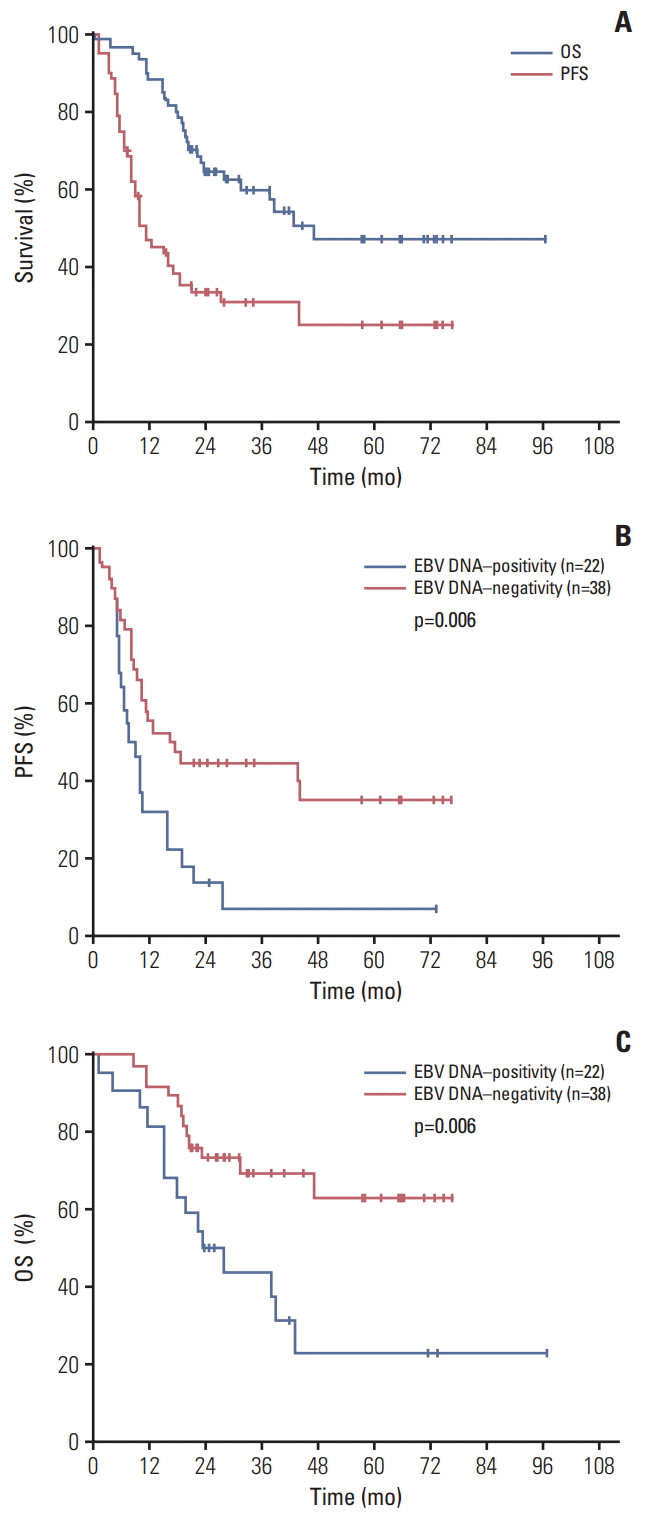
Fig. 2.
Sequential measurements of Epstein-Barr virus (EBV) copies number during therapy and follow-up of 22 EBV DNA‒positive angioimmunoblastic T-cell lymphoma patients and therapy response. DA-EPOCH, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin; PD, progressive disease; PR, partial response; CR, complete response; CRu, unconfirmed CR.
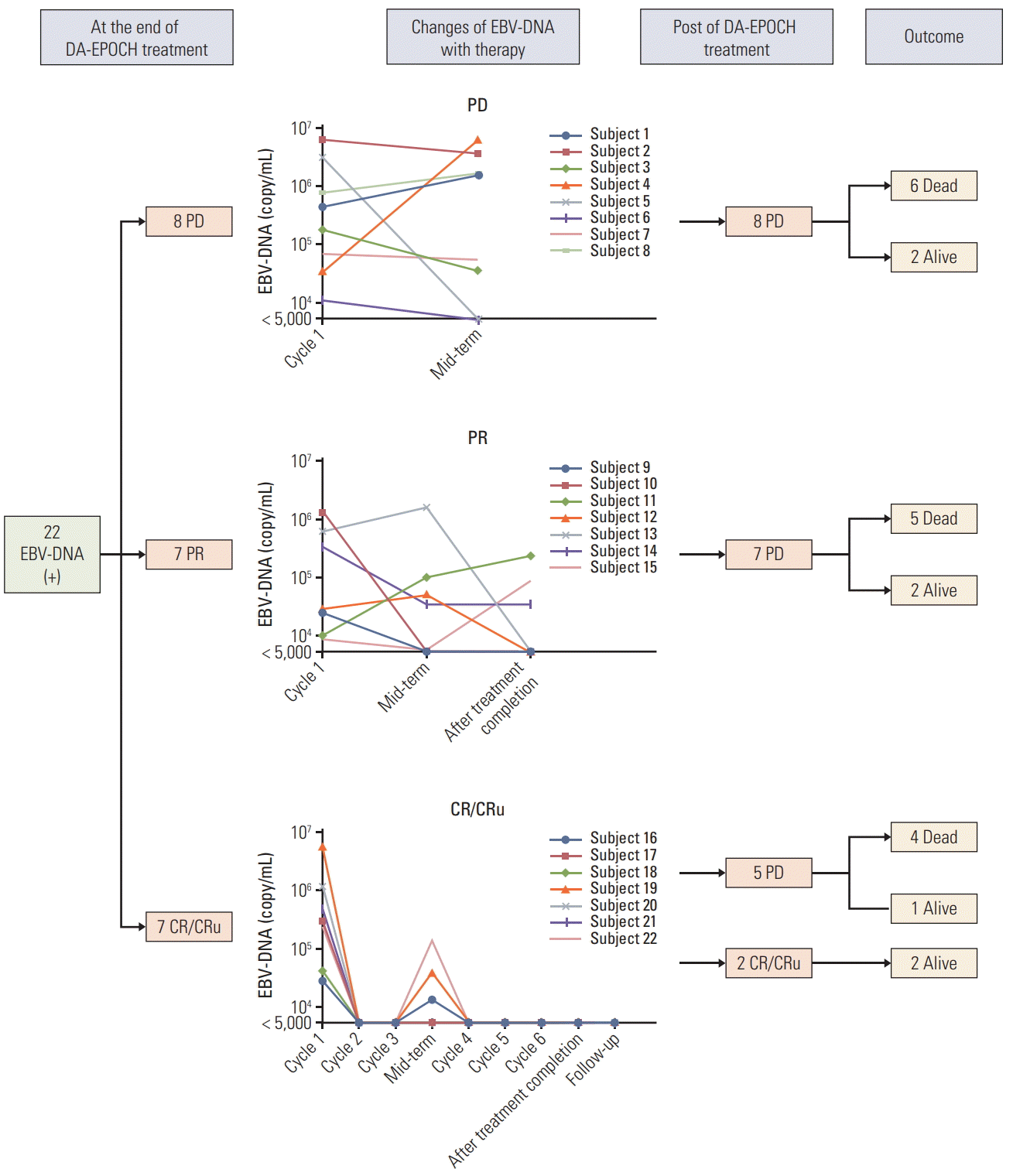
Table 1.
Clinical characteristics with the analysis of pretreatment EBV DNA
Table 2.
Univariate and multivariate Cox regression analyses of PFS and OS




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download