Abstract
Purpose
Dexrazoxane has been used as an effective cardioprotector against anthracycline cardiotoxicity. This study intended to analyze cardioprotective efficacy and secondary malignancy development, and elucidate risk factors for secondary malignancies in dexrazoxane-treated pediatric patients.
Materials and Methods
Data was collected from 15 hospitals in Korea. Patients who received any anthracyclines, and completed treatment without stem cell transplantation were included. For efficacy evaluation, the incidence of cardiac events and cardiac event-free survival rates were compared. Data about risk factors of secondary malignancies were collected.
Results
Data of total 1,453 cases were analyzed; dexrazoxane with every anthracyclines group (D group, 1,035 patients) and no dexrazoxane group (non-D group, 418 patients). Incidence of the reported cardiac events was not statistically different between two groups; however, the cardiac event-free survival rate of patients with more than 400 mg/m2 of anthracyclines was significantly higher in D group (91.2% vs. 80.1%, p=0.04). The 6-year cumulative incidence of secondary malignancy was not different between both groups after considering follow-up duration difference (non-D, 0.52%±0.37%; D, 0.60%±0.28%; p=0.55). The most influential risk factor for secondary malignancy was the duration of anthracycline administration according to multivariate analysis.
Conclusion
Dexrazoxane had an efficacy in lowering cardiac event-free survival rates in patients with higher cumulative anthracyclines. As a result of multivariate analysis for assessing risk factors of secondary malignancy, the occurrence of secondary malignancy was not related to dexrazoxane administration.
Anthracycline plays an important role in chemotherapy of various cancer types, including breast cancer, leukemia, and sarcoma so that about 50% of pediatric cancer patients are receiving anthracycline based chemotherapy [1]. However, cardiotoxicity is well known side effect of anthracycline, and leaves permanent cardiac damage in certain patients. The likelihood of anthracycline-related cardiotoxicity is reported to be up to 57%, and mortality rate due to these heart problems is reported as 8.2 times higher than that of the normal people [2]. In particular, the risk of late onset cardiotoxicity is reported to be higher in patients younger at the time of anthracycline administration, and received higher total cumulative anthracycline dose.
Because it is important to prevent late anthracycline induced cardiac event due to irreversibility, various attempts to prevent cardiac toxicity of anthracyclines were tried, such as changing administration methods or co-infusion of candidate cardio-protective agents. However, those methods were not effective in preventing cardiac toxicity only except dexrazoxane (Cardioxane) [1].
Dexrazoxane has been widely used to prevent cardiac toxicity after anthracycline administration including pediatric cancer patients, and showed clinically significant cardio-protective effects [3,4]. However, in a previous prospective study of Hodgkin’s disease, there was a claim that dexrazoxane might have increased the incidence of myelodysplastic syndrome (MDS) and secondary cancers [5].
However, on the contrary to the report with Hodgkin’s disease, following studies with various diseases and large numbers of patients showed that there was no difference in the occurrence of secondary malignancies (SMN) between patients administered dexrazoxane and the others [6-8]. In addition, there was no previous report about risk factor analysis including multivariate factors influencing the development of SMN. It is needed to verify whether SMN occurs in patients who received dexrazoxane, and whether other risk factors are associated with SMN. This study intended to analyze cardioprotective efficacy and SMN development related to dexrazoxane, and find out risk factors for SMN in Korean patients who received dexrazoxane.
This nationwide study was conducted in 15 institutions in Korea. The subjects for this study were selected from all pediatric cancer patients whose treatment was ended at the time of study initiation (November 2012). Among those, patients who had received anthracycline during their chemotherapy and did not receive hematopoietic stem cell transplantation were finally selected for the analysis. The patients were divided into two groups: (1) patients who used dexrazoxane with anthracyclines, and (2) patients who did not use the dexrazoxane. For the comparison with control group, patients who had received dexrazoxane with every dose of anthracyclines during their treatment regardless of the preceding risk factors for cardiotoxicity were included, and those who were administered dexrazoxane intermittently or only during selected schedules were excluded.
Various clinical and laboratory data were collected, which included patients’ demographics, diagnosis, chemotherapy history, dexrazoxane related data (total dose, date of first and last administration), any cardiac event, survival status and other late effects at the last follow up. Data reported to be risk factors of SMN were also collected including any congenital condition or hereditary syndrome, granulocyte colony-stimulating factor (G-CSF) administration and the details of radiation therapy. Irradiation on the thoraxinvolved fields (thorax, lung, craniospinal, mediastinum, etc.) might influence late cardiac function, thus radiation in those locations was considered as an independent factor for the analysis of late cardiac function. Cumulative doses of various chemotherapeutic agents including anthracyclines (daunorubicin, doxorubicin, idarubicin, epirubicin, and mitoxantrone), etoposide and cyclophosphamide were calculated. All anthracycline doses were converted to doxorubicin equivalent doses with the formula using doxorubicin equivalent dose conversion [9].
All the information was obtained using the electronic case report form developed by the Medical Research Collaborating Center (MRCC) in Seoul National University of Medicine, and was inputted by a designated researcher at each hospital. Data-management experts at MRCC performed quality-control checks to ensure accuracy of the recorded data, and audits were conducted thoroughly.
Baseline and demographic characteristics were summarized by standard descriptive statistics. Cumulative doses of chemotherapeutic agents and data related to dexrazoxane were summarized by descriptive statistics. For continuous data, basic statistical values were obtained, and T-test for parametric data and Wilcoxon rank sum test for nonparametric data were conducted. In case of categorical data, the frequencies and percentages were obtained, and Pearson's chi-square test or Fisher exact test were conducted for comparison between groups.
Events were defined as any relapse, death, or SMN. Cardiac events were defined as any cardiac abnormalities detected during and after the administration of anthracyclines including sudden cardiac death, and early cardiac toxicity. We defined early cardiotoxicity as which developed during treatment or first year after treatment completion, whereas late cardiotoxicity as which developed at least one year after therapy in cancer survivors [10,11]. Probabilities of event-free survival (EFS) and overall survival (OS) were estimated by Kaplan-Meier method, and survival differences were analyzed with log-rank test. Cox proportional hazard regression model was made after being adjusted for baseline and other factors which might influence on the outcome variables. Cardiac EFS was separately calculated for four groups which were divided according to each quartile values of the cumulative doses of anthracyclines and dexrazoxane.
The incidence of SMN and death in the two groups and unadjusted odds ratios were estimated with 95% confidence intervals. Considering the competing risks for death or relapse, cumulative incidence curves were calculated, and Fine and Gray models were used for the analysis of the difference between two groups. In addition, competing risk analysis with Fine and Gray model was conducted for the evaluation of risk factors for SMN. The correction factors for competing risk analysis were age, sex, diagnosis, G-CSF administration, hereditary or genetic syndrome, cumulative doses of chemotherapeutics, irradiation history, duration of anthracycline administration, duration between first anthracycline and last echocardiography, and total duration of chemotherapy.
All the statistical analysis was performed by biostatistics specialists at the MRCC in Seoul National University of Medicine, and SAS ver. 9.4 (SAS Institute Inc., Cary, NC) was used as a statistics program.
Data of total 1,788 patients from 15 institutions were collected, and data of total 1,453 patients were analyzed for the study (S1 Fig., S2 Table). The date of initial diagnosis of all patients were ranged from August 1996 to September 2012. Dexrazoxane group (D group) included 1,035 patients, and non-dexrazoxane group (non-D group) included 418 patients. Median age at diagnosis was 5 years in non-D group, and 6 years in D group (Table 1). Age and sex proportions were not statistically different between two groups, but follow-up duration was longer in non-D group, as median 124.3 months comparing with 59.4 months in D group (p < 0.01).
Diagnosis of patients was various as shown in Table 1. In D group, there were acute lymphoblastic leukemia (26.5%), non-Hodgkin's lymphoma (16.0%), and osteosarcoma (12.2%) in the order of proportion, and acute lymphoblastic leukemia (42.6%), non-Hodgkin's lymphoma (18.7%) and neuroblastoma (6.5%) in non-D group. The distribution of diagnosis was statistically different between two groups (p < 0.01).
We collected data of hereditary syndrome or genetic disease in all subjects (Table 1). There were 12 patients who were diagnosed with hereditary syndrome or genetic diseases; two (0.5%) patients in non-D group and 10 (1.0%) patients in D group, respectively.
Cumulative doses of chemotherapeutic agents are shown in Table 2. The median cumulative doses of etoposide were 1,830 mg/m2 in non-D group and 1.82 g/m2 in D group (p=0.52). Cumulative doses of cyclophosphamide were significantly higher in the D group, as median cumulative doses were 4,030 mg/m2 in D group and 2,570 mg/m2 in non-D group (p < 0.01). The data about anthracyclines doses was collected in 1,443 patients, and the dose of total cumulative anthracyclines was significantly higher in D group, as 210 mg/m2 in D group and 150 mg/m2 in non-D group, respectively (p < 0.01). Among anthracyclines, cumulative doses of doxorubicin and idarubicin were significantly higher in the D group.
The median duration of entire chemotherapy was 652 days in non-D group and 366 days in D group, and total duration of chemotherapy was significantly longer in non-D group (p < 0.01) (Table 2), and the median total duration of anthracycline administration was 161 days in non-D group and 168 days in D group (p=0.90). The median age of first anthracycline administration was 5 years in non-D group and 6 years in D group, respectively (p=0.73). The median dose of dexrazoxane was 2,390 mg/m2, and the median total duration of dexrazoxane administration was 167 days (range, 1 to 2,974 days).
Routine G-CSF administration during neutropenia was more frequent in D group (86.0%) than in non-D group (71.2%) (p < 0.01), and more patients in non-D group (32.1%) received radiotherapy (p < 0.01) (Table 2). The total dose of radiation was significantly higher in D group (p=0.01); however, radiation fields were various and the number of patients who received radiation therapy on thorax-related fields was not significantly different between two groups (p=0.48).
Cardiac events in 1,452 patients until the time of data collection were collected. A total of 75 cardiac events occurred, and there were 16 cases (3.8%) in non-D group, and 59 cases (5.7%) in D group (p=0.24) (S3 Table). Among all cardiac events, asymptomatic ventricular dysfunction in echocardiography was the most common cardiac manifestation (3 cases in non-D group, 26 cases in D group). According to the time of cardiotoxicity, early cardiotoxicity occurred in 13 cases of non-D group, and 52 cases of D group.
The cardiac EFS rate was 95.4% in non-D group, and 93.4% in D group. There was no significant difference in EFS after considering the corrections factors (p=0.09) (Fig. 1A). According to the total anthracyclines cumulative doses, there were 204 patients who received more than 400 mg/m2 of anthracyclines (32 patients in non-D group, 172 patients in D group). There were 6 cardiac events in non-D group, and 12 events in D group (S4 Table). When analyzing the cardiac EFS rates according to the total cumulative anthracycline doses, there was significant difference between non-D group and D group of patients who received more than 400 mg/m2, as 80.1% and 91.2% (p=0.04) (Fig. 1B).
Data of SMN was available in 1,224 patients (non-D group 344 patients and D group 880 patients), and there were 4 and 12 patients who developed SMN or MDS in non-D group and D group, respectively (Table 3). The median latency from initial diagnosis to SMN was 5.7 years (range, 1.3 to 13.9 years). The most common SMN was acute myeloid leukemia (AML), as total five patients developed AML, and osteosarcoma was developed in three patients. The follow up duration of both groups were significantly different, so the cumulative incidence of both groups were compared at the same time points. The 6-year cumulative incidence rate of SMN in all patients was 0.67±0.24%. The rates of SMN were 0.52%±0.37% in non-D group and 0.60%±0.28% in D group, respectively (Fig. 2A), and the cumulative incidence rate after considering correction factors was not statistically different between two groups (p=0.55). When patients with Hodgkin lymphoma were analyzed (non-D group 12 patients, and D group 34 patients), the 6-year cumulative incidence rate of SMN was 0% in non-D group and 3.1%±2.9% in D group, and the difference between two groups was not significant (p=0.56) (Fig. 2B).
Risk factors affecting the occurrence of SMN after considering relapse and death as competing risks were analyzed with Fine and Gray model, and the results are shown in Table 4. According to the univariate analysis with significant level of 5%, irradiation history, higher cumulative doses of etoposide, cyclophosphamide and anthracyclines, longer duration of anthracycline, shorter time after last anthracycline, and longer duration of chemotherapy were related with statistically significant higher hazard ratio of the occurrence of SMN. The result of multivariate analysis showed that the incidence of SMN was not increased due to dexrazoxane, but increased approximately 1.05 times in response to every 1-month prolongation of anthracycline administration (p < 0.01) (Table 4). Whereas, the incidence of SMN was reduced by about 0.99 times as every month after last anthracycline went by (p < 0.01).
In this study, multivariate analysis for assessing risk factors of SMN in pediatric cancer patients who received dexrazoxane was conducted, and there was no increased incidence or risk of SMN in those patients.
The mechanism of anthracycline’s early cardiotoxicity is known to be related to free radical injury, contributing to the formation of reactive oxygen species and leading to the apoptosis of cardiomyocyte and intracellular damage [12]. On the other hand, the mechanism of delayed cardiotoxicity in the long-term survivors is multifactorial including myocardial mitochondria related apoptosis which results in metabolic remodeling of heart [13]. However, delayed cardiotoxicity presents as overt clinical manifestations such as heart failure only in extreme cases, and only slowly progressing ventricular abnormalities are detected in many cases [13].
For assessing cardiotoxicity in pediatric cancer patients, cardiac event has been counted as one of the markers for cardiotoxicity [3,14]. A cumulative dose of anthracycline has been known as the most significant risk factor for cardiac dysfunction. The incidence of heart failure was approximately 3.0% in patients receiving a cumulative dose of doxorubicin of 400 mg/m2, 7.5% at doses of 550 mg/m2, and 18.0% at doses of 700 mg/m2 [15]. In our study, although there was no significant difference in EFS between two groups and the median follow up duration was significantly longer in non-D group, patients who received more than 400 mg/m2 of cumulative anthracyclines and dexrazoxane showed a slightly higher cardiac EFS than the other patients without dexrazoxane. These results might be related to the relatively low incidence of clinical cardiologic events in patients with less than 400 mg/m2 of anthracycline, thus the protective effect would be apparent only in patients receiving higher amounts of anthracyclines. Longer follow-up would be needed considering late cardiotoxicity especially in patients with short treatment off period.
Despite of the cardioprotective effects of dexrazoxane, dexrazoxane was reported to be related with increased risk of SMN. Tebbi et al. [5] reported the results of the Pediatric Oncology Group study with Hodgkin lymphoma, in which patients were randomly assigned to receive dexrazoxane to evaluate its cardiopulmonary protective effect. The authors found an increased incidence of SMN after a median follow-up time of 4.8 years, especially AML or MDS, in the dexrazoxane-received patients.
However, many refutations against the Tebbi’s report have been followed. Since in that article, risk factors of SMN such as cumulative doses of etoposide, cyclophosphamide, and doxorubicin or G-CSF administration were not considered as confounding factors in the analysis. Also, many studies in other patient groups have not shown the same results [6-8,13].
In our study, multiple risk factors known to affect SMN development were collected. In the multivariate analysis, longer duration of anthracycline, and shorter time after last anthracycline were related with statistically significant higher hazard ratio of the occurrence of SMN. There was no significant risk factor related to AML or MDS, specifically. There was no data related to the association of SMN and duration of certain chemotherapeutic agents. Considering the final adjusted hazard ratios in our study were relatively low as 1.05 and 0.99, our final multivariate factors could not be the most powerful risk factors for SMN. Further analysis could elucidate more influential risks factors for SMN in these patients group.
Risk factors for SMN are known to be multifactorial. Chemotherapy agents known to be carcinogenic are the alkylating agents, topoisomerase II agents, and anthracyclines [16]. In addition, the cumulative dose, the schedule of chemotherapy administration, and the use of multiple drugs all significantly impact the risk of developing SMN [16]. In our study, patient No. 2 in Table 3 developed papillary thyroid cancer within radiation fields. Although there were cases where the irradiated fields and location of SMN were not related, radiation itself has been reported as one of the risk factors for SMN in childhood cancer patients [17-19]. Secondary osteosarcoma was developed in three retinoblastoma patients. The risk of secondary bone tumors in retinoblastoma survivors is higher in patients with bilateral disease or germline mutations in RB gene. In addition, radiation therapy could also increase the risk of secondary osteosarcoma in retinoblastoma patients [20].
When patients with Hodgkin disease were analyzed separately, dexrazoxane was not a significant risk factor for SMN and the cumulative incidence of SMN was not significantly different regardless of dexrazoxane. In the study of the Childhood, Adolescent and Young Adult Cancer Survivors Research Program of British Columbia which analyzed long term results of 442 Hodgkin lymphoma survivors, radiation therapy (hazard ratio, 2.7), and female sex (hazard ratio, 1.8) were significant risk factors for SMN in multivariate analysis [21]. To analyze risk factors for SMN in Hodgkin disease, many factors other than dexrazoxane should be considered.
This study has several limitations. First, there would be possibility of selection bias due to retrospective design although data management and analysis were conducted by medical statistics specialists. Second, the follow up duration was variable, and there were patients with insufficient follow-up duration for late complication analysis. Especially, the follow up duration of D group was significantly shorter than non-D group so that cardiac events or SMN results might be changed after longer follow-up. Third, there could be other risk factors for SMN development such as cancer predisposing genetic factors and other alkylating agents including ifosfamide. In addition, specific medical conditions which might cause cardiac events other than anthracycline could not be considered because of the limitation of retrospective design.
In conclusion, dexrazoxane had an efficacy in the prevention of lowering cardiac EFS rates in patients with higher cumulative anthracyclines. According to the multivariate analysis, the occurrence of SMN was not related to dexrazoxane. Given the efficacy of dexrazoxane as a cardioprotectant, dexrazoxane could be used in anthracycline-containing pediatric regimens.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
ACKNOWLEDGMENTS
This research was supported by a grant (12172MFDS231) from the Ministry of Food and Drug Safety, Republic of Korea.
References
1. Harake D, Franco VI, Henkel JM, Miller TL, Lipshultz SE. Cardiotoxicity in childhood cancer survivors: strategies for prevention and management. Future Cardiol. 2012; 8:647–70.

2. Brewster DH, Clark D, Hopkins L, Bauer J, Wild SH, Edgar AB, et al. Subsequent hospitalisation experience of 5-year survivors of childhood, adolescent, and young adult cancer in Scotland: a population based, retrospective cohort study. Br J Cancer. 2014; 110:1342–50.

3. Choi HS, Park ES, Kang HJ, Shin HY, Noh CI, Yun YS, et al. Dexrazoxane for preventing anthracycline cardiotoxicity in children with solid tumors. J Korean Med Sci. 2010; 25:1336–42.

4. Barry EV, Vrooman LM, Dahlberg SE, Neuberg DS, Asselin BL, Athale UH, et al. Absence of secondary malignant neoplasms in children with high-risk acute lymphoblastic leukemia treated with dexrazoxane. J Clin Oncol. 2008; 26:1106–11.

5. Tebbi CK, London WB, Friedman D, Villaluna D, De Alarcon PA, Constine LS, et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J Clin Oncol. 2007; 25:493–500.

6. Walker DM, Fisher BT, Seif AE, Huang YS, Torp K, Li Y, et al. Dexrazoxane use in pediatric patients with acute lymphoblastic or myeloid leukemia from 1999 and 2009: analysis of a national cohort of patients in the Pediatric Health Information Systems database. Pediatr Blood Cancer. 2013; 60:616–20.

7. Vrooman LM, Neuberg DS, Stevenson KE, Asselin BL, Athale UH, Clavell L, et al. The low incidence of secondary acute myelogenous leukaemia in children and adolescents treated with dexrazoxane for acute lymphoblastic leukaemia: a report from the Dana-Farber Cancer Institute ALL Consortium. Eur J Cancer. 2011; 47:1373–9.

8. Seif AE, Walker DM, Li Y, Huang YS, Kavcic M, Torp K, et al. Dexrazoxane exposure and risk of secondary acute myeloid leukemia in pediatric oncology patients. Pediatr Blood Cancer. 2015; 62:704–9.

9. Blanco JG, Sun CL, Landier W, Chen L, Esparza-Duran D, Leisenring W, et al. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes: a report from the Children's Oncology Group. J Clin Oncol. 2012; 30:1415–21.
10. Florescu M, Cinteza M, Vinereanu D. Chemotherapy-induced cardiotoxicity. Maedica (Buchar). 2013; 8:59–67.
11. Kremer LC, van Dalen EC, Offringa M, Ottenkamp J, Voute PA. Anthracycline-induced clinical heart failure in a cohort of 607 children: long-term follow-up study. J Clin Oncol. 2001; 19:191–6.

12. Lipshultz SE, Adams MJ. Cardiotoxicity after childhood cancer: beginning with the end in mind. J Clin Oncol. 2010; 28:1276–81.

13. Lipshultz SE, Scully RE, Lipsitz SR, Sallan SE, Silverman LB, Miller TL, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol. 2010; 11:950–61.

14. Mozdzanowska D, Wozniewski M. Radiotherapy and anthracyclines: cardiovascular toxicity. Contemp Oncol (Pozn). 2015; 19:93–7.
15. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003; 97:2869–79.
17. Hawkins MM, Wilson LM, Stovall MA, Marsden HB, Potok MH, Kingston JE, et al. Epipodophyllotoxins, alkylating agents, and radiation and risk of secondary leukaemia after childhood cancer. BMJ. 1992; 304:951–8.

18. Haddy N, Le Deley MC, Samand A, Diallo I, Guerin S, Guibout C, et al. Role of radiotherapy and chemotherapy in the risk of secondary leukaemia after a solid tumour in childhood. Eur J Cancer. 2006; 42:2757–64.

19. Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010; 102:1083–95.

20. Choi DK, Helenowski I, Hijiya N. Secondary malignancies in pediatric cancer survivors: perspectives and review of the literature. Int J Cancer. 2014; 135:1764–73.

21. Bhuller KS, Zhang Y, Li D, Sehn LH, Goddard K, McBride ML, et al. Late mortality, secondary malignancy and hospitalisation in teenage and young adult survivors of Hodgkin lymphoma: report of the Childhood/Adolescent/Young Adult Cancer Survivors Research Program and the BC Cancer Agency Centre for Lymphoid Cancer. Br J Haematol. 2016; 172:757–68.

Fig. 1.
Cardiac event-free survival (EFS) of all patients (A), patients who received more than 400 mg/m2 of total cumulative anthracyclines (B). (A) The cardiac EFS rate was 95.4% in non-dexrazoxane group (non-D group) (n=416), and 93.4% in dexrazoxane group (D group) (n=1,034) (p=0.09), (B) but there was significant difference in cardiac EFS between non-D group (n=32) and D group of patients (n=172) who received more than 400 mg/m2, as 80.1% and 91.2% (p=0.04).
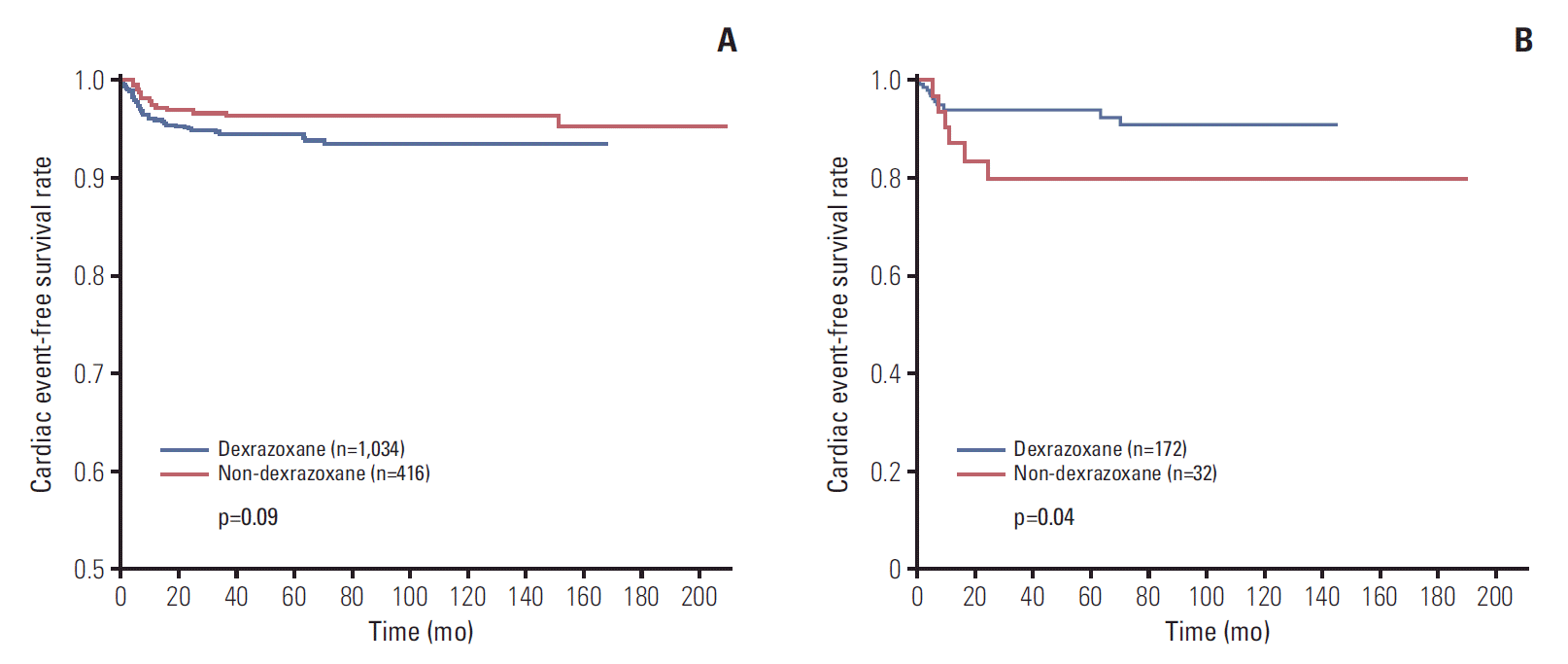
Fig. 2.
The 6-year cumulative incidence rate of secondary malignancy in all patients (n=1,224) (A), patients with Hodgkin disease (n=46) (B). (A) The 6-year cumulative incidence rate of secondary malignancies (SMN) in all patients was 0.67±0.24%. The rates of SMN were 0.52%±0.37% in non-dexrazoxane group (non-D group) (n=880) and 0.60%±0.28% in dexrazoxane group (D group) (n=344) (p=0.55). (B) When patients with Hodgkin lymphoma were analyzed, the rates of SMN was 0% in non-D group (n=12) and 3.1%±2.9% in D group (n=34) (p=0.56).
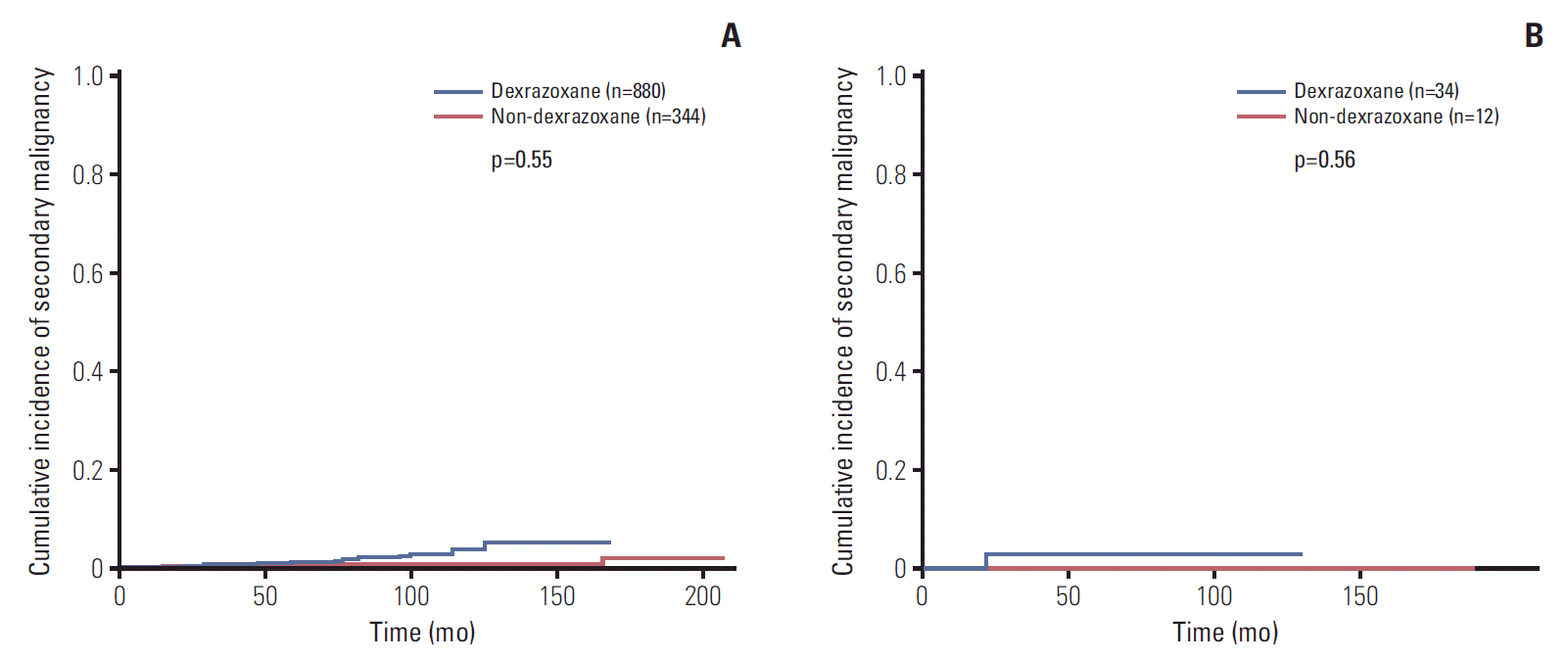
Fig. 3.
Survival rates of all patients. (A) Overall survival rate of 1,035 patients in dexrazoxane group (D group) was 87.4%, and that of 418 patients in non-dexrazoxane group (non-D group) was 86.3% (p=0.06). (B) Event-free survival rate was 80.3% in D group (n=1,035) and 80.4% in non-D group (n=418) (p=0.64).
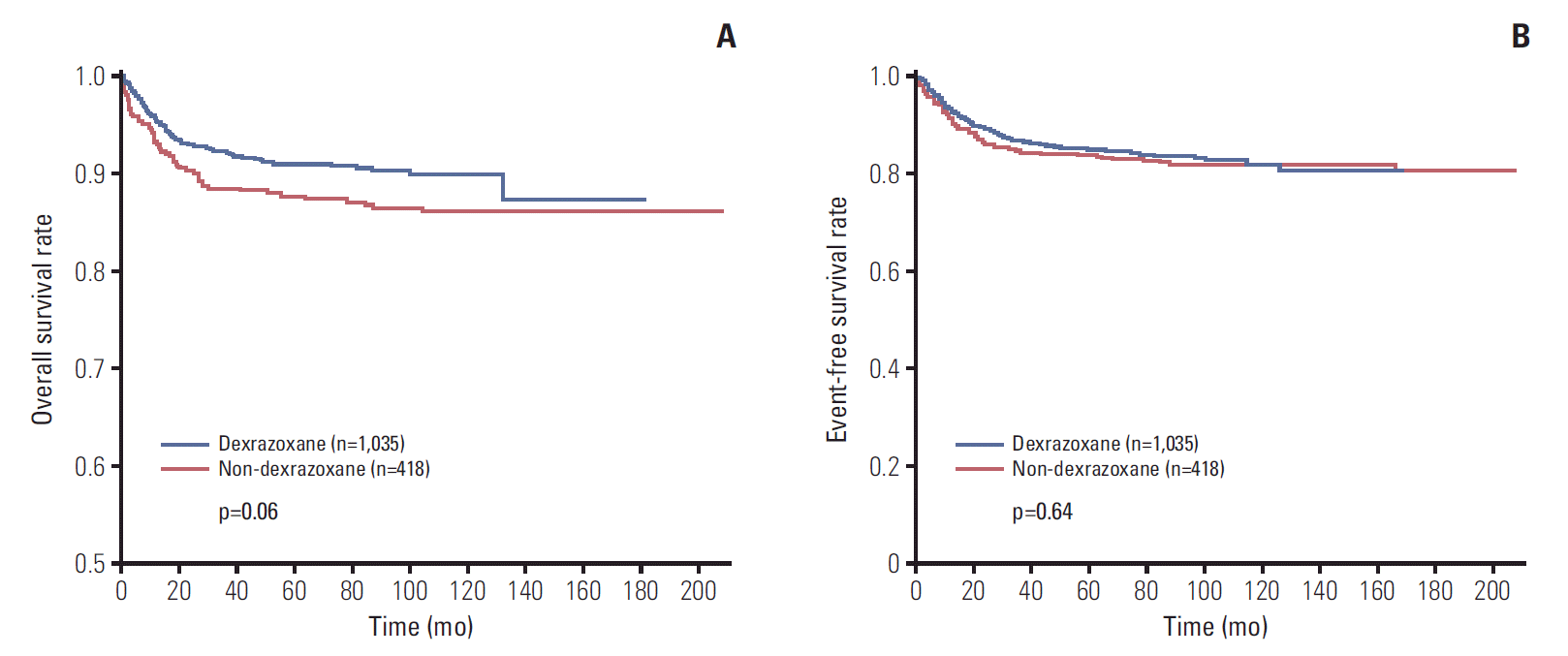
Table 1.
Clinical characteristics of all patients
Table 2.
Cumulative doses of chemotherapeutics and details of treatment
|
Non-dexrazoxane (n=418) |
Dexrazoxane (n=1,035) |
p-value | |||
|---|---|---|---|---|---|
| No. (%) | Median (range) | No. (%) | Median (range) | ||
| Cumulative doses of chemotherapeutics (mg/m2) | |||||
| Etoposide | 122 | 1,830 (120-6,340) | 447 | 1,820 (40-15,430) | 0.52 |
| Cyclophosphmide | 297 | 2,570 (310-35,370) | 718 | 4,030 (4-74,110) | < 0.01 |
| Total anthracyclinesa) | 411b) | 150 (15.4-665.1) | 1,032c) | 210 (17-753.9) | < 0.01 |
| Daunomycin (daunorubicin) | 164 | 75 (18-347) | 304 | 80 (18.4-392) | 0.20 |
| Doxorubicin (adriamycin) | 364 | 120 (15-691) | 909 | 180 (17.3-665) | < 0.01 |
| Idarubicin | 27 | 50 (6-113) | 80 | 60 (10.7-248) | 0.03 |
| Epirubicin | 1 | 208 | 13 | 100 (31.1-1,091) | 1.00 |
| Mitoxantrone | 15 | 40 (16-100) | 35 | 40 (12-55) | 0.73 |
| Total duration of chemotherapy (day)d) | - | 652 (0-2,655) | - | 366 (1-3,444) | < 0.01 |
| Total duration of anthracycline (day)d) | - | 161 (0-1,889) | - | 168 (0-2,974) | 0.90 |
| Age at first anthracycline (yr) | - | 5 (0-26) | - | 6 (0-21) | 0.73 |
| Total dose of dexrazoxane (mg/m2) | - | - | - | 2,390 (125-36,989) | < 0.01 |
| Total duration of dexrazoxane (day)d) | - | - | - | 167 (1-2,974) | < 0.01 |
| G-CSF administration during neutropenia | 297 (71.2) | - | 890 (86.0) | - | < 0.01 |
| Radiotherapy | 134 (32.1) | - | 201 (19.4) | - | < 0.01 |
| Radiotherapy on thorax | 9 (2.2) | - | 29 (2.8) | - | 0.48 |
| Radiation dose (Gy) | - | 18 (2-63) | - | 22.5 (0.45-147) | 0.01 |
Table 3.
Characteristics of patients with secondary malignancies
Table 4.
Analysis of the risk factors for secondary malignancy




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download