1. Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumors of the digestive system. 4th ed. Lyon: IARC Press;2010.
2. Donahue TR, Reber HA. Surgical management of pancreatic cancer: pancreaticoduodenectomy. Semin Oncol. 2015; 42:98–109.
3. Bali MA, Metens T, Denolin V, Delhaye M, Demetter P, Closset J, et al. Tumoral and nontumoral pancreas: correlation between quantitative dynamic contrast-enhanced MR imaging and histopathologic parameters. Radiology. 2011; 261:456–66.

4. Balachandran A, Bhosale PR, Charnsangavej C, Tamm EP. Imaging of pancreatic neoplasms. Surg Oncol Clin N Am. 2014; 23:751–88.

5. Bartsch DK, Gress TM, Langer P. Familial pancreatic cancer: current knowledge. Nat Rev Gastroenterol Hepatol. 2012; 9:445–53.
6. Takai E, Yachida S, Shimizu K, Furuse J, Kubo E, Ohmoto A, et al. Germline mutations in Japanese familial pancreatic cancer patients. Oncotarget. 2016; 7:74227–35.

7. Cho JH, Bang S, Park SW, Chung JB, Song SY. BRCA2 mutations as a universal risk factor for pancreatic cancer has a limited role in Korean ethnic group. Pancreas. 2008; 36:337–40.

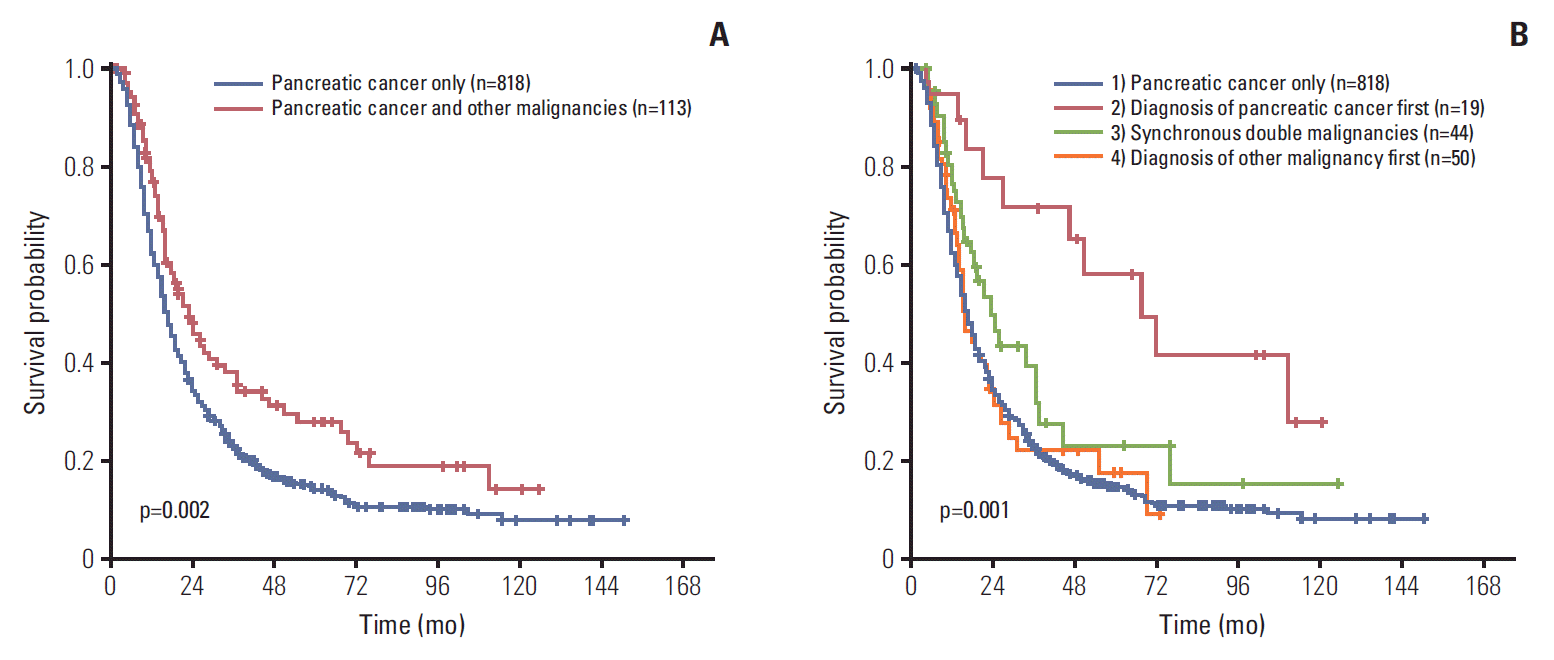
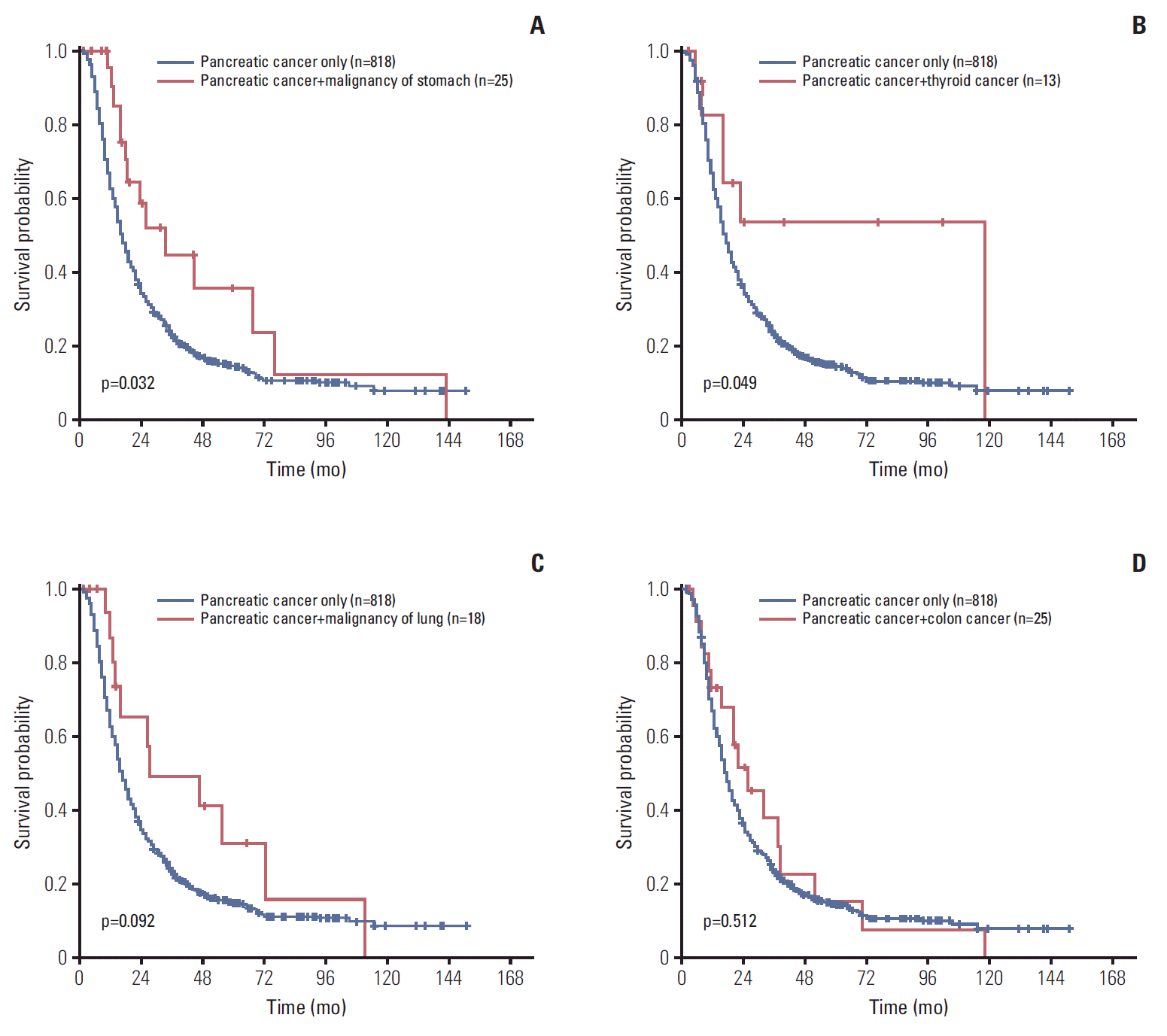
8. Eriguchi N, Aoyagi S, Hara M, Okuda K, Tamae T, Fukuda S, et al. Synchronous or metachronous double cancers of the pancreas and other organs: report on 12 cases. Surg Today. 2000; 30:718–21.

9. Gerdes B, Ziegler A, Ramaswamy A, Wild A, Langer P, Bartsch DK. Multiple primaries in pancreatic cancer patients: indicator of a genetic predisposition? Int J Epidemiol. 2000; 29:999–1003.

10. Moertel CG. Multiple primary malignant neoplasms: historical perspectives. Cancer. 1977; 40(4 Suppl):1786–92.
11. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti AE. AJCC cancer staging handbook. 7th ed. New York: Springer;2010.
12. Tagawa T, Ito K, Fukuzawa K, Okamoto T, Fujinaga A, Kawasaki T, et al. Surgical outcomes of non-small cell lung cancer in patients with a history of pancreaticobiliary cancer. Anticancer Res. 2017; 37:3307–9.

13. Ghothim M, Havlik R, Skalicky P, Klos D, Vrba R, Straznicka J, et al. Synchronous cancer duplicities of pancreas and stomach/kidney and their surgical treatment. Rozhl Chir. 2015; 94:251–5.
14. Muller SA, Pahernik S, Hinz U, Martin DJ, Wente MN, Hackert T, et al. Renal tumors and second primary pancreatic tumors: a relationship with clinical impact? Patient Saf Surg. 2012; 6:18.

15. Kamisawa T, Isawa T, Egawa N, Tsuruta K, Okamoto A, Kawamura T, et al. Study on pancreatic cancer associated with other primary malignancies. Suizo. 1993; 8:164–9.
16. Makino TK, Takahashi S, Maruyama H, Kohara T, Yokose Y, Emi Y, et al. Clinicopathology of pancreatic cancer analyzed from annual of the pathological autopsy cases in Japan. Tan To Sui. 1984; 5:761–8.
17. Yoshimori M, Tajiri H, Nakamura K, Kishi K, Ozaki H. Clinical significance of multiple cancers including pancreatic cancer: report on 12 cases. Gan No Rinsho. 1982; 28:165–7.
18. Maruchi N, Brian D, Ludwig J, Elveback LR, Kurland LT. Cancer of the pancreas in Olmsted County, Minnesota, 1935-1974. Mayo Clin Proc. 1979; 54:245–9.
19. Cubilla A, Fitzgerald PJ. Pancreas cancer. I. Duct adenocarcinoma. A clinical-pathologic study of 380 patients. Pathol Annu. 1978; 13 Pt 1:241–89.
20. Kasumi F, Togo S, Ota T, Takagi K, Kato H. Study of cases with double carcinoma of the stomach and pancreas. Gan No Rinsho. 1977; 23:1306–14.
21. Moertel CG, Dockerty MB, Baggenstoss AH. Multiple primary malignant neoplasm. I. Introduction and presentation of data. Cancer. 1961; 14:221–30.
22. Warren S, Gates O. Multiple primary malignant tumors: a survey of the literature and a statistical study. Am J Cancer. 1932; 16:1358–414.
23. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010; 138:2044–58.

24. Jun SY, Lee EJ, Kim MJ, Chun SM, Bae YK, Hong SU, et al. Lynch syndrome-related small intestinal adenocarcinomas. Oncotarget. 2017; 8:21483–500.

25. Malesci A, Laghi L, Bianchi P, Delconte G, Randolph A, Torri V, et al. Reduced likelihood of metastases in patients with microsatellite-unstable colorectal cancer. Clin Cancer Res. 2007; 13:3831–9.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download