Introduction
Materials and Methods
1. ESCC cell lines
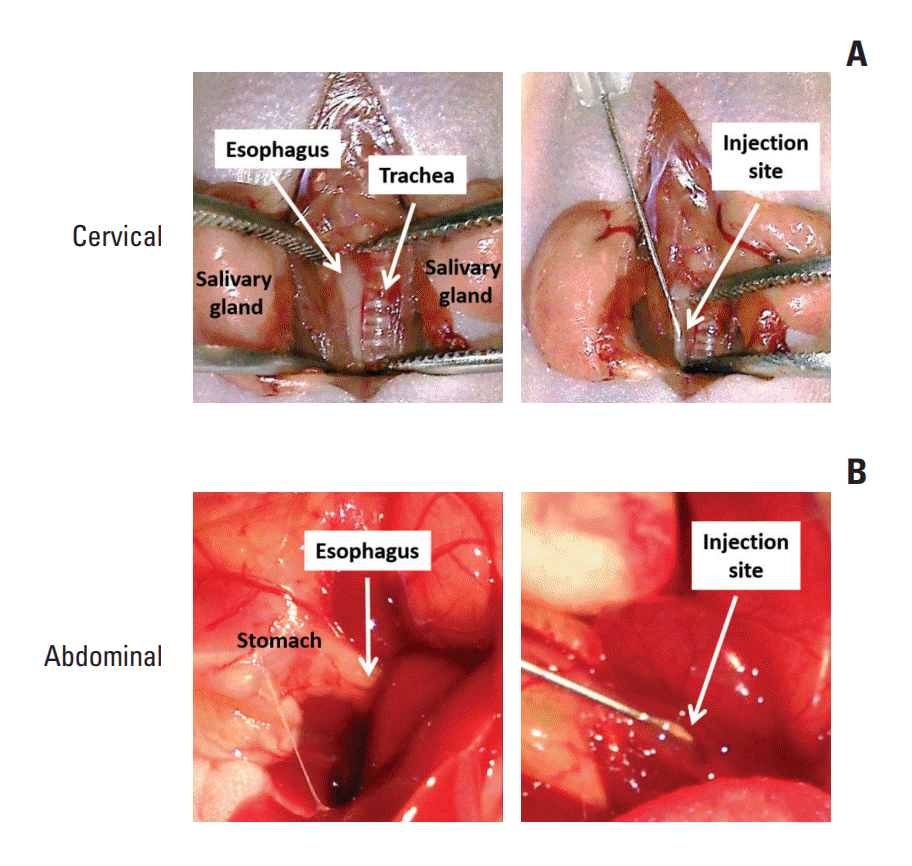
2. Nude mice
3. Orthotopic tumor xenograft models
4. Curcumin analogues synthesis
5. MTT cell proliferation assay
6. Cell cycle analysis
7. Western blotting
8. In vivo tumor growth assay
9. Statistical analyses
10. Ethical statement
Results
1. Tumor xenograft establishment in mouse esophagus
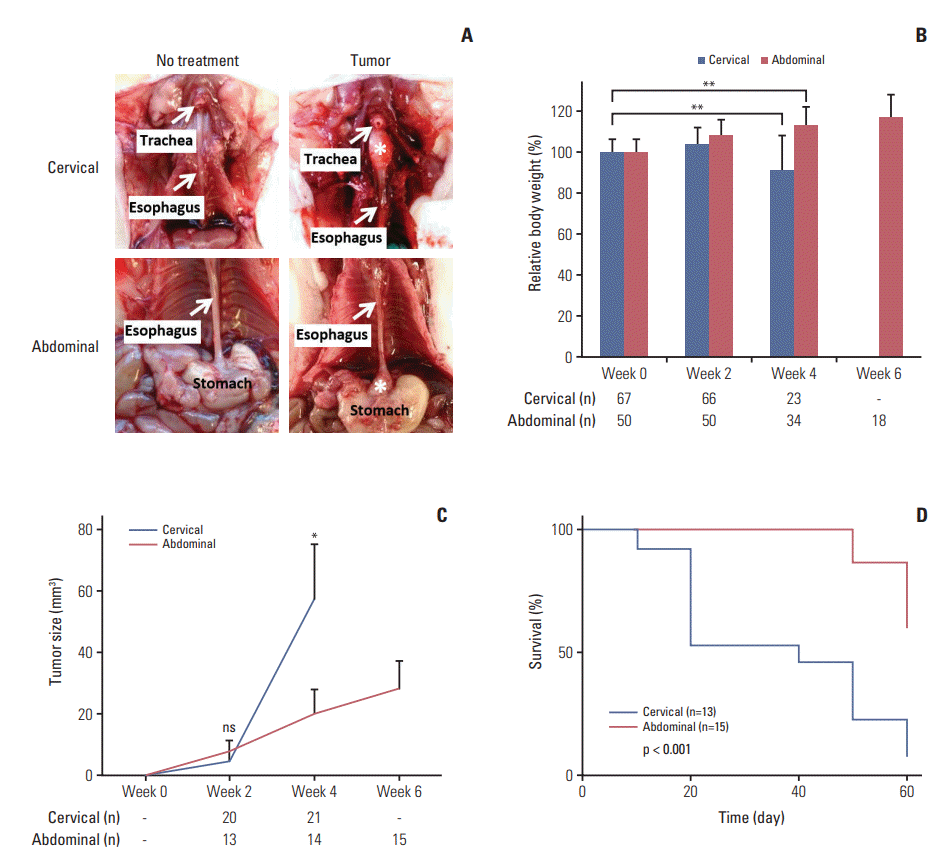 | Fig. 2.Comparison between orthotopic tumor xenograft models at the cervical and abdominal esophagus. (A) Photos taken at week 4 (cervical) or week 6 (abdominal) after SLMT-1 esophageal squamous cell carcinoma cell injection into the esophageal wall show successful establishment of tumor xenografts, marked by asterisk, at the cervical or abdominal esophagus. (B) Mice bearing tumor xenograft at the cervical esophagus experienced a significant body weight reduction in 4 weeks’ time, whereas a gradual increase was noticed in those with tumor xenograft at the abdominal esophagus. Bars indicate mean±standard deviation (SD). **p < 0.01. (C) When the tumor xenografts were dissected for tumor size measurement every two weeks, we observed faster growth of tumor xenografts at the cervical esophagus compared to those at the abdominal esophagus. Error bars indicate SD. *p < 0.05. ns, statistically not significant. (D) Kaplan-Meier survival curve analysis revealed poorer survival in mice bearing tumor xenograft at the cervical esophagus than those with tumor xenograft at the abdominal esophagus. p < 0.001. n, animal number. |
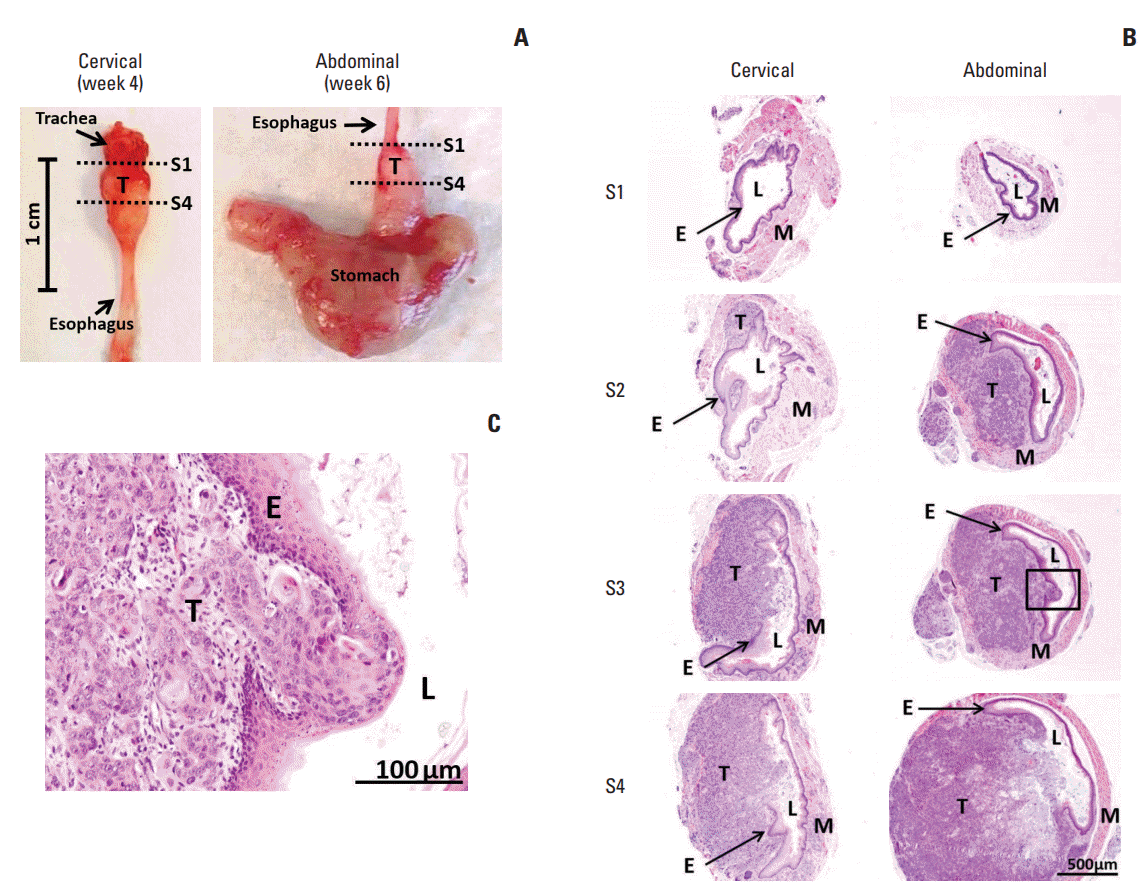 | Fig. 3.Histological images of SLMT-1-derived tumor xenografts at the cervical and abdominal esophagus. (A) Mouse esophagus with tumor xenograft was dissected to prepare serial sections from proximal location S1 to distal location S4, as indicated by dotted lines. (B) Hematoxylin and eosin staining was used to reveal the histology of the representative sections at location S1, S2, S3, and S4. S1, no observable tumor; S2, tumor centered in the muscularis propria and submucosa of the esophageal wall; S3 and S4, tumor invasion into the esophageal lumen and at the same time causing luminal stricture (×40). (C) The tumor invasive front can be clearly seen in a high magnification image of squared area in S3 section (×200). E, epithelium of esophagus; L, esophageal lumen; M, muscular layer of esophagus; T, tumor. |
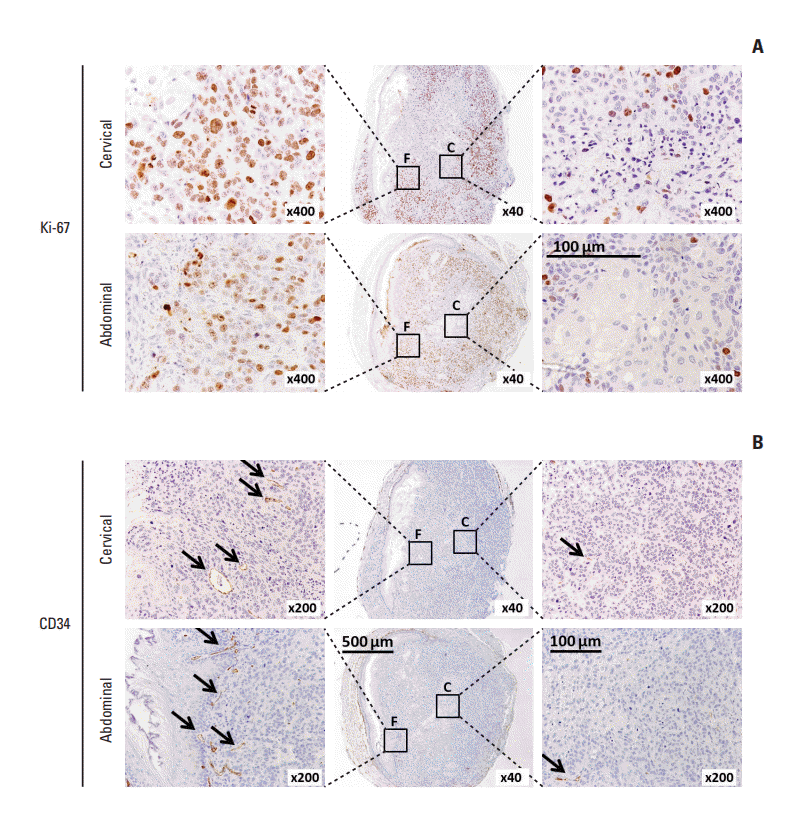 | Fig. 4.Immunohistochemical staining of Ki-67 and CD34 in orthotopic tumor xenografts. Tumor xenografts formed at the
cervical and abdominal esophagus using SLMT-1 esophageal squamous cell carcinoma cells were subjected to immunohistochemistry by staining with two different molecular markers, i.e., Ki-67 for proliferating cells (A) and CD34 for tumor vessels (B). Most of the positively stained cells (arrows) were concentrated at the tumor periphery (area F) rather than the tumor center (area C). |
2. In vitro anti-tumor effects of curcumin analogues in ESCC
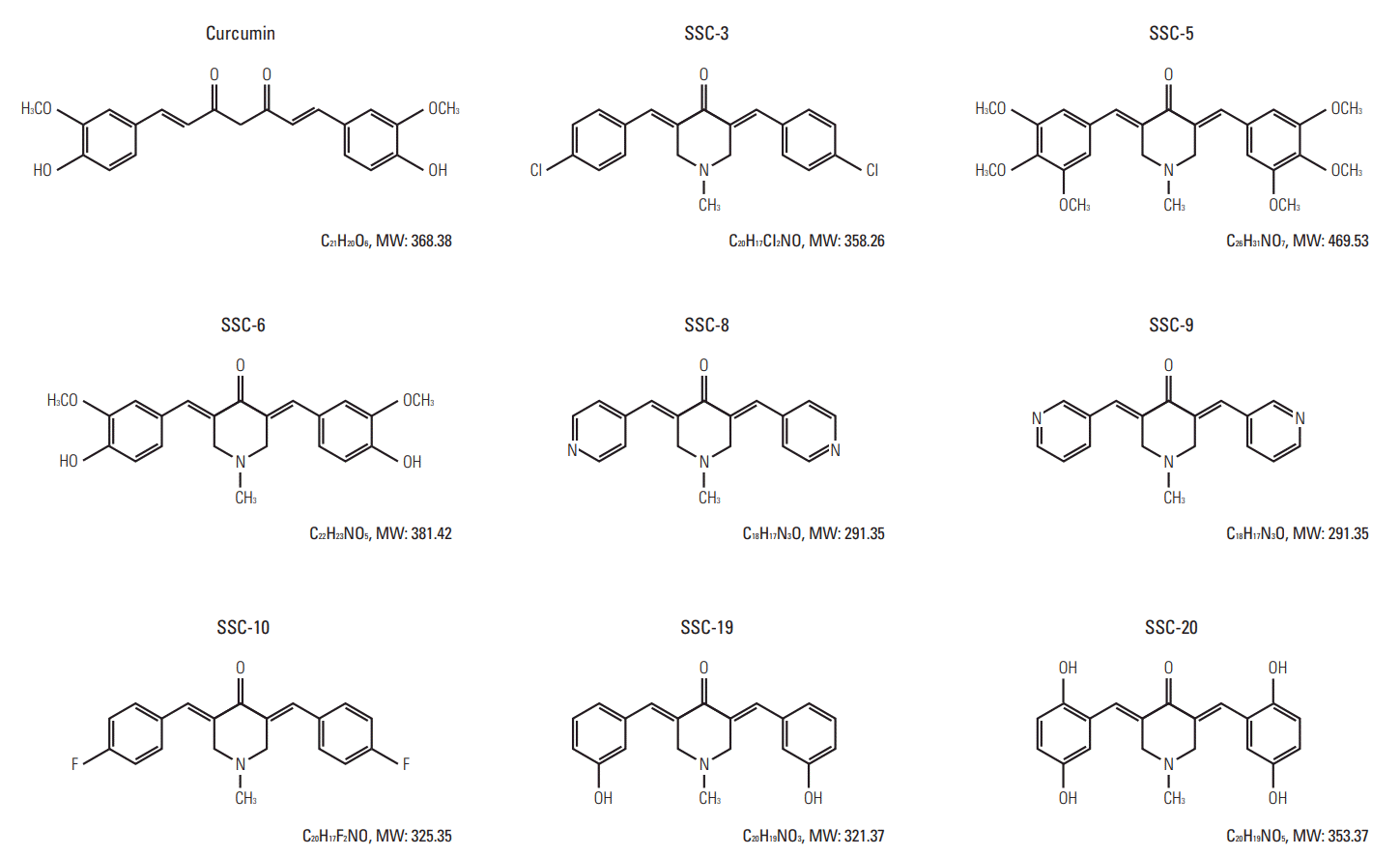 | Fig. 5.Molecular structures of curcumin and its analogues. Curcumin, 1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione; SSC-3, 3,5-Bis-(4-chlorobenzylidene)-1-methylpiperidin-4-one; SSC-5, 1-Methyl-3,5-bis-(3,4,5-trimethoxybenzylidene)piperidin-4-one; SSC-6, 3,5-Bis-(4-hydroxy-3-methoxybenzylidene)-1-methylpiperidin-4-one; SSC-8, 1-Methyl-3, 5-bispyridin-4-ylmethylenepiperidin-4-one; SSC-9, 1-Methyl-3,5-bispyridin-3-ylmethylenepiperidin-4-one; SSC-10, 3,5-Bis-(4-fluorobenzylidene)-1-methylpiperidin-4-one; SSC-19, 3,5-Bis-(3-hydroxybenzylidene)-1-methylpiperidin-4-one; SSC-20, 3,5-Bis-(2,5-dihydroxybenzylidene)-1-methylpiperidin-4-one. |
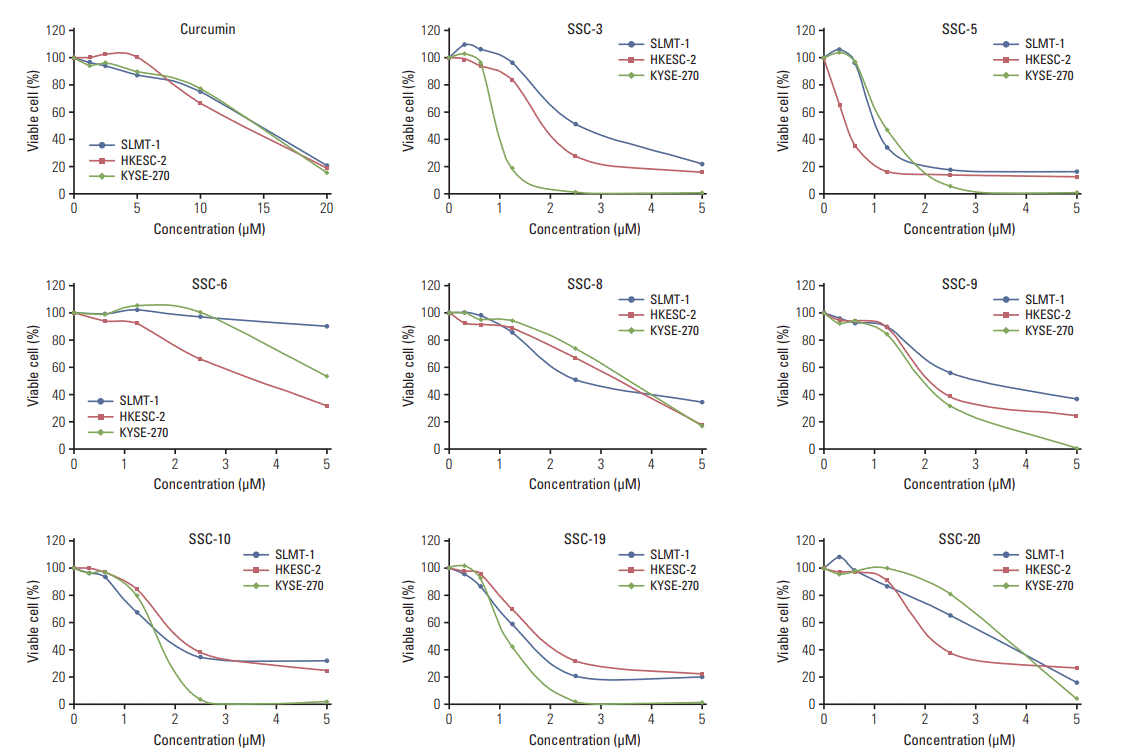 | Fig. 6.Curcumin analogues exhibit potent growth inhibiting effect in cultured esophageal squamous cell carcinoma (ESCC) cells. In vitro MTT cell proliferation assay was performed in three ESCC cell lines SLMT-1, HKESC-2, and KYSE-270 after treatment with curcumin (0-20 μM) and its analogues (0-5 μM). Based on the IC50 obtained after a 48-hour treatment period, curcumin analogues demonstrated more potent growth inhibiting effect in ESCC cells than curcumin. Representative results are shown. |
Table 1.
Table 2.
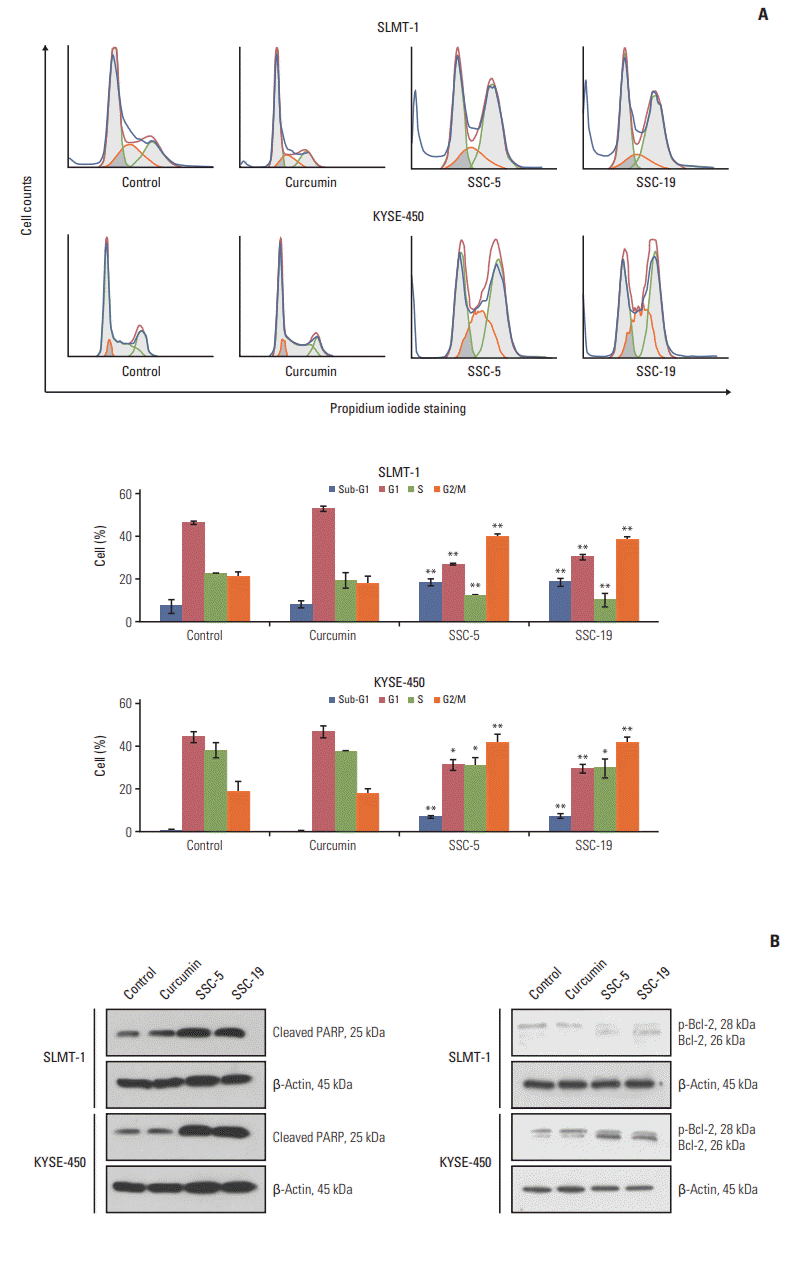 | Fig. 7.SSC-5 and SSC-19 induce cell cycle arrest and apoptosis in cultured esophageal squamous cell carcinoma (ESCC)
cells. (A) Flow cytometry analysis revealed the DNA content of SLMT-1 and KYSE-450 ESCC cells after treatment with dissolved in dimethyl sulfoxide (control), curcumin, SSC-5, and SSC-19. Representative flow cytometric profiles are shown. Treatment with SSC-5 and SSC-19 resulted in a significant induction of cells in the sub-G1 and G2/M phase of the cell cycle. Bars indicate mean±standard deviation. *p < 0.05, **p < 0.01. (B) Western blotting experiment showed an obvious induction of cleaved poly(ADPribose) polymerase (PARP) and a de-phosphorylation of Bcl-2 in ESCC cells after treatment with SSC-5 and SSC-19. β-Actin was used as a loading control. |
3. Tumor growth and invasion inhibition capacity of SSC-5 in ESCC in vivo
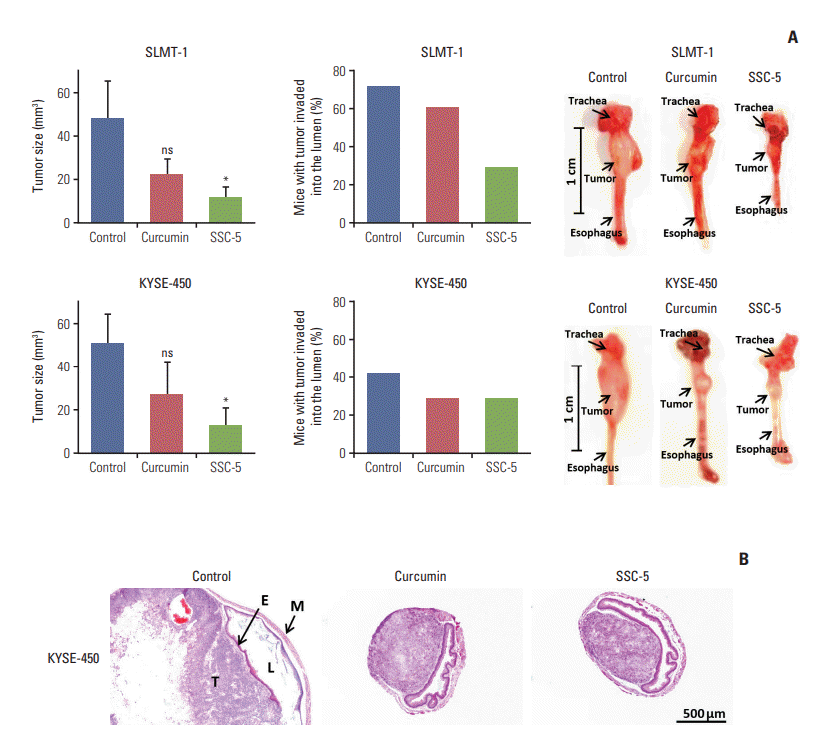 | Fig. 8.SSC-5 inhibits growth and invasion abilities of orthotopic tumor xenografts. Tumor xenografts were established at the cervical esophagus in nude mice using SLMT-1 and KYSE-450 esophageal squamous cell carcinoma (ESCC) cells. Tumorbearing mice were randomly divided into three groups for a 2-week treatment with dissolved in dimethyl sulfoxide (control), curcumin and SSC-5. (A) Treatment with SSC-5 resulted in a significant tumor size reduction (left panel) and a drop in the percentage of mice with tumor invasion into the esophageal lumen (middle panel). Right panel shows the representative photographs from each experimental group. Data are presented in mean±standard error of mean. ns, statistically not significant. *p < 0.05. (B) Representative histological images show tumor invasion into the esophageal lumen in control group, while the occurrence of such event was reduced under the treatment with curcumin or SSC-5 (×40). E, epithelium of esophagus; L, esophageal lumen; M, muscular layer of esophagus; T, tumor. |
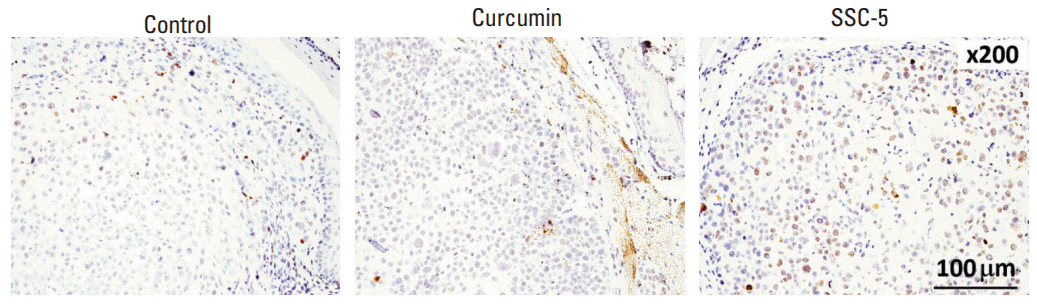 | Fig. 9.SSC-5 treatment induces expression level of cleaved poly (ADP-ribose) polymerase (PARP) in orthotopic tumor xenografts. KYSE-450 esophageal squamous cell carcinoma cells were used to establish tumor xenografts at the cervical esophagus in nude mice. Treatment of these mice with SSC-5 induced the expression level of cleaved PARP in tumor xenografts, while a lower level was detected in tumor xenografts from curcumin treatment and control group. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download