1. Grande E, Bolos MV, Arriola E. Targeting oncogenic ALK: a promising strategy for cancer treatment. Mol Cancer Ther. 2011; 10:569–79.

2. Gridelli C, Peters S, Sgambato A, Casaluce F, Adjei AA, Ciardiello F. ALK inhibitors in the treatment of advanced NSCLC. Cancer Treat Rev. 2014; 40:300–6.

3. Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009; 27:4247–53.

4. Shaw AT, Engelman JA. ALK in lung cancer: past, present, and future. J Clin Oncol. 2013; 31:1105–11.
5. Camidge DR, Bang YJ, Kwak EL, Iafrate AJ, Varella-Garcia M, Fox SB, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012; 13:1011–9.

6. Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010; 363:1693–703.
7. Kim D, Ahn M, Yang P, Liu X, De Pas T, Crino L, et al. Updated results of a global phase II study with crizotinib in advanced ALK-positive non-small cell lung cancer. Ann Oncol. 2012; 23:402.
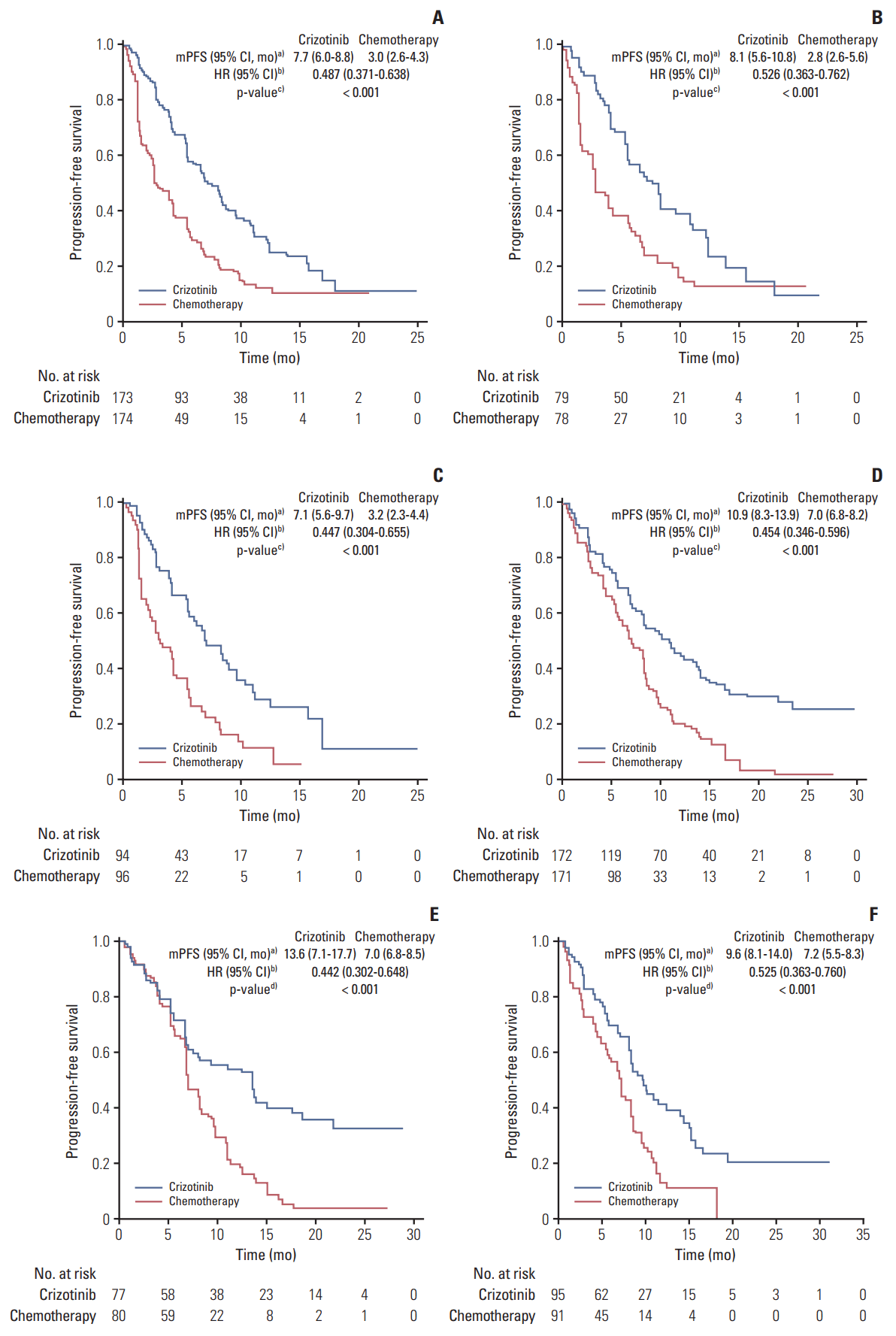
8. Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013; 368:2385–94.

9. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014; 371:2167–77.

10. Lu S, Mok T, Lu Y, Zhou J, Shi Y, Sriuranpong V, et al. Phase 3 study of first-line crizotinib vs pemetrexed-cisplatin/carboplatin (PCC) in East Asian patients (pts) with ALK+ advanced non-squamous non-small cell lung cancer (NSCLC). J Clin Oncol. 2016; 34(15 Suppl):Abstr.

11. Ou SH, Salgia R, Clark J, Kwak EL, Camidge DR, Maki R, et al. Comparison of crizotinib (PF-02341066) pharmacokinetics between Asian and non-Asian patients with advanced malignancies. J Thorac Oncol. 2010; 5:S382.
12. Park K, Kim JH, Cho EK, Kang JH, Shih JY, Zimmermann AH, et al. East Asian subgroup analysis of a randomized, double-blind, phase 3 study of docetaxel and ramucirumab versus docetaxel and placebo in the treatment of stage IV non-small cell lung cancer following disease progression after one prior platinum-based therapy (REVEL). Cancer Res Treat. 2016; 48:1177–86.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download