Introduction
Materials and Methods
1. Data sources
2. Case definitions
3. Statistical analysis
Table 1.
| Variable | Cutaneous melanoma | Squamous cell carcinoma | Basal cell carcinoma | p-value |
|---|---|---|---|---|
| Total | 6,207 (100) | 12,516 (100) | 22,283 (100) | |
| Age at diagnosis (yr) | ||||
| Mean±SD | 60.5±15.7 | 72.2±13.7 | 67.3±12.6 | < 0.001a) |
| < 40 | 604 (9.7) | 296 (2.4) | 605 (2.7) | |
| 40-59 | 2,097 (33.8) | 1,842 (14.7) | 4,922 (22.1) | |
| 60-79 | 2,928 (47.2) | 6,178 (49.3) | 13,185 (49.2) | |
| ≥ 80 | 578 (9.3) | 4,200 (33.6) | 3,571 (16.0) | |
| Sex | ||||
| Male | 3,003 (48.4) | 5,286 (42.2) | 9,621 (43.2) | < 0.001b) |
| Female | 3,204 (51.6) | 7,230 (57.8) | 12,662 (56.8) | |
| Year at diagnosis | ||||
| 1999-2002 | 941 (15.2) | 1,513 (12.1) | 2,108 (9.4) | < 0.001b) |
| 2003-2006 | 1,369 (22.0) | 2,294 (18.3) | 3,856 (17.3) | |
| 2007-2010 | 1,779 (28.7) | 3,609 (28.8) | 6,677 (30.0) | |
| 2011-2014 | 2,118 (34.1) | 5,100 (40.8) | 9,642 (43.3) |
Results
1. Baseline characteristics
Table 2.
2. Incidence rate of melanoma and non-melanoma skin cancer
Table 3.
| Category |
Study period |
AAPC (95% CI, %) | ||||
|---|---|---|---|---|---|---|
| Overall (1999-2014) | 1999-2002 | 2003-2006 | 2007-2010 | 2011-2014 | ||
| Men | ||||||
| Cutaneous melanoma | 0.66 | 0.51 | 0.66 | 0.72 | 0.67 | 3.0 (0.8 to 5.3)* |
| Sun-exposed region | 0.16 | 0.11 | 0.15 | 0.16 | 0.15 | 5.1 (0.8 to 9.5)* |
| Non‒sun-exposed region | 0.41 | 0.30 | 0.39 | 0.43 | 0.44 | 2.4 (0.7 to 4.3)* |
| Squamous cell carcinoma | 1.34 | 0.95 | 1.08 | 1.29 | 1.28 | 3.3 (2.6 to 4.0)* |
| Sun-exposed region | 0.79 | 0.53 | 0.64 | 0.80 | 0.94 | 4.5 (3.4 to 5.6)* |
| Non‒sun-exposed region | 0.35 | 0.32 | 0.35 | 0.34 | 0.35 | 1.1 (‒0.2 to 2.4) |
| Basal cell carcinoma | 2.45 | 1.18 | 1.63 | 2.44 | 2.35 | 8.0 (6.0 to 10.1)* |
| Sun-exposed region | 1.92 | 1.01 | 1.44 | 2.12 | 2.56 | 8.2 (6.0 to 10.5)* |
| Non‒sun-exposed region | 0.21 | 0.11 | 0.14 | 0.21 | 0.27 | 8.3 (5.9 to 10.9)* |
| Women | ||||||
| Cutaneous melanoma | 0.58 | 0.43 | 0.54 | 0.62 | 0.60 | 3.5 (2.4 to 4.6)* |
| Sun-exposed region | 0.15 | 0.09 | 0.14 | 0.13 | 0.15 | 6.4 (2.5 to 10.4)* |
| Non‒sun-exposed region | 0.35 | 0.24 | 0.31 | 0.35 | 0.42 | 4.8 (4.2 to 6.4)* |
| Squamous cell carcinoma | 1.04 | 0.59 | 0.85 | 1.13 | 1.11 | 6.8 (5.3 to 8.4)* |
| Sun-exposed region | 0.81 | 0.42 | 0.61 | 0.88 | 1.03 | 6.7 (2.9 to 10.7)* |
| Non‒sun-exposed region | 0.18 | 0.14 | 0.17 | 0.17 | 0.19 | 3.4 (2.1 to 4.8)* |
| Basal cell carcinoma | 2.07 | 0.98 | 1.67 | 2.34 | 2.25 | 9.0 (7.5 to 10.4)* |
| Sun-exposed region | 1.92 | 0.91 | 1.57 | 2.12 | 2.60 | 9.3 (7.6 to 10.9)* |
| Non‒sun-exposed region | 0.14 | 0.07 | 0.09 | 0.15 | 0.18 | 11.4 (7.3 to 15.7)* |
The age-standardized incidence rates are presented as incidence cases per 100,000 people using Segi’s world standard population as standard population. Skin cancer sites were categorized into sun-exposed sites (face and neck: ICD-10 codes C430-C433, C440-C443) and sites that are not usually exposed to sun (trunk and lower limbs: ICD-10 codes C435, C437, C445, C447).
The unit for AAPC was expressed as % per 1 year. AAPC, average annual percent change; CI, confidence interval; ICD-10, International Classification of Diseases, 10th revision.
3. Joinpoint regression analysis for trends in incidence of melanoma and non-melanoma skin cancer
Fig. 1.
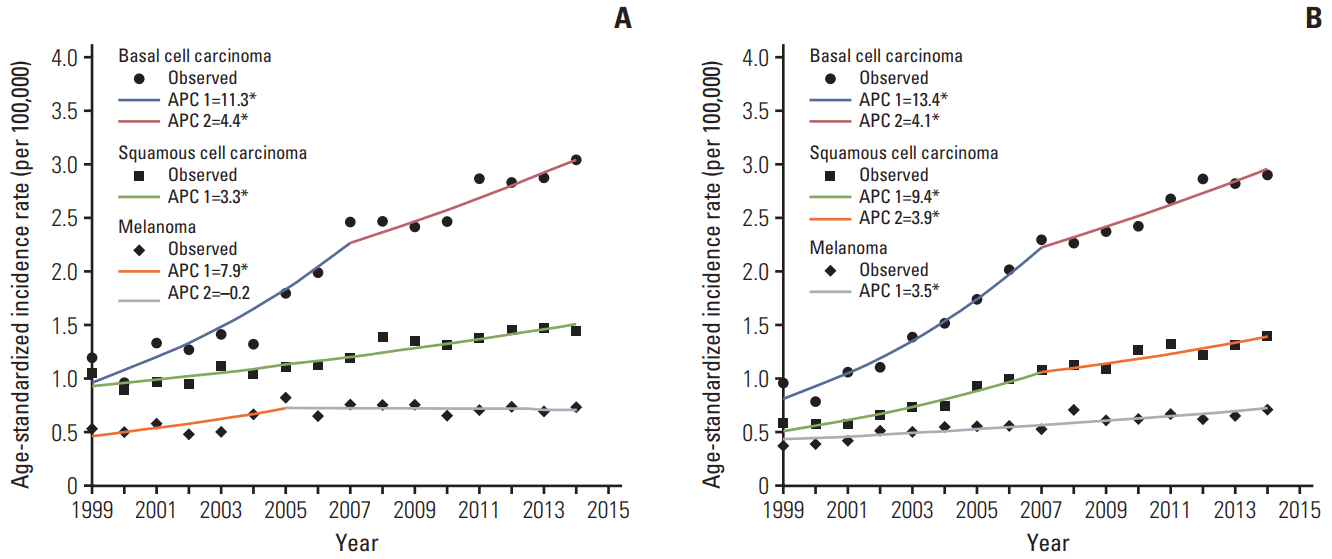
4. Trends in the incidence rate of melanoma and nonmelanoma by subsites
Table 4.
| Category |
Trend 1 |
Trend 2 |
Trend 3 |
|||
|---|---|---|---|---|---|---|
| Year | APC (95% CI, %) | Year | APC (95% CI, %) | Year | APC (95% CI, %) | |
| Men | ||||||
| Cutaneous melanoma | 1999-2005 | 7.9 (2.4 to 13.7)* | 2005-2014 | ‒0.2 (‒2.3 to 2.0) | - | - |
| Sun-exposed region | 1999-2006 | 14.5 (5.4 to 24.3)* | 2006-2014 | ‒2.5 (‒7.2 to 2.4) | - | - |
| Non‒sun-exposed region | 1999-2014 | 2.4 (0.7 to 4.3)* | - | - | - | - |
| Squamous cell carcinoma | 1999-2014 | 3.3 (2.6 to 4.0)* | - | - | - | - |
| Sun-exposed region | 1999-2014 | 4.5 (3.4 to 5.6)* | - | - | - | - |
| Non‒sun-exposed region | 1999-2014 | 1.1 (‒0.2 to 2.4) | - | - | - | - |
| Basal cell carcinoma | 1999-2007 | 11.3 (7.6 to 15.1)* | 2007-2014 | 4.4 (1.9 to 7.0)* | - | - |
| Sun-exposed region | 1999-2007 | 11.5 (7.4 to 15.7)* | 2007-2014 | 4.7 (1.9 to 7.5)* | - | - |
| Non‒sun-exposed region | 1999-2014 | 8.3 (5.9 to 10.9)* | - | - | - | - |
| Women | ||||||
| Cutaneous melanoma | 1999-2014 | 3.5 (2.4 to 4.6)* | - | - | - | - |
| Sun-exposed region | 1999-2003 | 21.7 (5.4 to 40.5)* | 2003-2014 | 1.3 (‒0.9 to 3.5) | - | - |
| Non‒sun-exposed region | 1999-2014 | 4.8 (3.2 to 6.4)* | - | - | - | - |
| Squamous cell carcinoma | 1999-2007 | 9.4 (6.7 to 12.3)* | 2007-2014 | 3.9 (1.9 to 6.0)* | - | - |
| Sun-exposed region | 1999-2001 | ‒3.6 (‒27.1 to 27.4) | 2001-2007 | 13.8 (8.5 to 19.3)* | 2007-2014 | 4.0 (2.1 to 5.9)* |
| Non‒sun-exposed region | 1999-2014 | 3.4 (2.1 to 4.8)* | - | - | - | - |
| Basal cell carcinoma | 1999-2007 | 13.4 (10.7 to 16.1)* | 2007-2014 | 4.1 (2.3 to 6.0)* | - | - |
| Sun-exposed region | 1999-2006 | 14.6 (10.9 to 18.4)* | 2007-2014 | 4.8 (3.2 to 6.4)* | - | - |
| Non‒sun-exposed region | 1999-2009 | 15.6 (10.1 to 21.4)* | 2009-2014 | 3.5 (‒4.5 to 12.1) | - | - |
The age-standardized incidence rates are presented as incidence cases per 100,000 people using Segi’s world standard population as standard population. Joinpoint regression analysis was used to determine whether there were significant changes in trends in age-standardized incidence rates for the period between 1999 and 2014. Skin cancer sites were categorized into sun-exposed sites (face and neck: ICD-10 codes C430-C433, C440-C443) and sites that are not usually exposed to sun (trunk and lower limbs: ICD-10 codes C435, C437, C445, C447). The unit for APC was expressed as % change per year. APC, annual percent change; CI, confidence interval; ICD-10, International Classification of Diseases, 10th revision.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download