Abstract
Purpose
The aim of this study was to estimate the burden of breast cancer that can be attributed to rapid lifestyle changes in South Korea in 2013-2030.
Materials and Methods
An age-period-cohort model was used to estimate the incidence and mortality. The Global Burden of Disease Study Group methodwas used to calculate the years of life lost and years lived with disability in breast cancer patients using a nationwide cancer registry. The population attributable riskswere calculated using meta-analyzed relative risk ratios and by assessing the prevalence of risk factors.
Results
Women’s reproductive/lifestyle changes, including advanced maternal age at first childbirth (from 37 to 85 disability-adjusted life years [DALYs] per 100,000 person-years), total period of breastfeeding (from 22 to 46 DALYs per 100,000 person-years), obesity (from 37 to 61 DALYs per 100,000 person-years), alcohol consumption (from 19 to 39 DALYs per 100,000 person-years), oral contraceptive use (from 18 to 27 DALYs per 100,000 person-years), and hormone replacement therapy use (from 2 to 3 DALYs per 100,000 person-years) were identified as factors likely to increase the burden of breast cancer from 2013 to 2030. Approximately, 34.2% to 44.3% of the burden of breast cancer could be avoidable in 2030 with reduction in reproductive/lifestyle risk factors.
Breast cancer is the second most common cancer among Korean females and has been increasing during the last decade [1]. According to the National Cancer Information Center, breast cancer accounts for 19.3% of all cancers among women [2], with an annual percentage change of 5.9% [1]. This increasing trend in breast cancer is probably related to changes in reproductive/lifestyle factors secondary to the rapid development and westernization of Korea, such as more advanced maternal age at first pregnancy, oral contraceptive (OC) use, hormone replacement therapy (HRT) use, obesity, and alcohol consumption [3]. Moreover, Korea is rapidly becoming an aged society, and survival from cancer, particularly breast cancer, is becoming common (92.0%). Furthermore, the 5-year survival rate is increasing with advanced medical technology and breast cancer screening programs [2]. As the incidence and survival rates of breast cancer are high and consistently increasing, the burden of breast cancer is becoming increasingly heavy. Furthermore, South Korea will be the first country to have an average life expectancy higher than 90 years by 2030 according to a previous study [4]. The growing burden of disease will be affected by the aging population, especially if people live an extended period in poor health. Nevertheless, most researchers have investigated only the cancer incidence or mortality for evaluating cancer burden, which do not reflect the quality of life [5].
To evaluate the quality of life of cancer patients, the World Health Organization and the Global Burden of Disease Study Group (GBD) developed an indicator, known as disabilityadjusted life years (DALYs), which considers both the time lost by premature death and time lived with disability [6]. DALYs can refer to the lost time of healthy life. DALYs are widely used to estimate the burden of cancer as a reflection of the quality of life, to confirm cost-effectiveness, and to compare cancer burdens across nations [7].
As mentioned above, to our knowledge, most studies on breast cancer burden have focused on breast cancer incidence or mortality as the study outcome, and did not evaluate the quality of life of breast cancer patients [5,8]. Further, the burden of cancer was measured using only the years of life lost (YLL) in some studies [9]. Kunnavil et al. [10] assessed the estimated burden of breast cancer through DALYs in 2016, 2021, and 2026 in India; however, they did not consider risk factors of breast cancer. Asadzadeh Vostakolaei et al. [11] considered the effect of women’s lifestyle changes on the future burden of breast cancer; however, the burden of breast cancer was measured as the change in breast cancer incidence in their study. Therefore, in the present study, we attempted to assess the effects of women’s lifestyle changes on the future burden of breast cancer using DALYs.
Reproductive risk factors (i.e., advanced age at first childbirth, early menarche, late menopause, and no or short breastfeeding duration) and lifestyle risk factors (i.e., inactivity, obesity, alcohol consumption, OC use, and HRT use) are responsible for a considerable proportion of breast cancer risk [12]. Unfortunately, the rapid westernization and economic growth has led to rapid changes in the reproductive and lifestyle risk factors of breast cancer in Korean women [13]. However, most reproductive and lifestyle risk factors of breast cancer are modifiable, which means that women can take actions to reduce the risk of breast cancer [14]. For example, the risk of breast cancer can be minimized by undergoing the first childbirth at an earlier age, losing weight, or reducing alcohol consumption.
The aim of this study was to estimate the trends in the burden of breast cancer and the effects of modifiable risk factor changes on the future burden of breast cancer. The findings of this study will be helpful for determining target groups for intervention and for providing evidence for resource allocation to reduce the burden of breast cancer. Furthermore, predicting the future burden may help policy makers plan for the rapidly changing burden of breast cancer. Moreover, assessing the potential effect of modifiable risk factors is essential for determining public health needs and developing strategies and policies for preventing breast cancer.
This study included all Korean women aged > 20 years who were identified using the Korea Central Cancer Registry data (1999-2013) (16,812,720 in 1999, 19,953,695 in 2013). This registry was established by the Ministry of Health and Welfare and has collected cancer statistics since 1999.
The methods used to predict the trends of the burden of breast cancer and the effects of modifiable risk factor changes on the burden of breast cancer in Korea during 2013-2030 are summarized below. There are several methods for determining the future projection of cancer incidence and mortality, such as simple linear regression, log linear regression, the GBD model, and age-period-cohort (APC) model. In order to predict the future cancer more accurately, we applied the Nordpred APC model using the R-package to evaluate the effects of age, period, and birth cohort on breast cancer incidence and mortality in 1999-2013 and projected the future trends for these effects for 2030. The APC model has the following several strengths: it consists of three interdependent time dimensions, including age (age at diagnosis), period (diagnosis year), and cohort (birth year); uses the power 5 link function to decrease exponential changes; and assumes that the overall past trends will continue in the future [15]. The Nordpred APC model is defined as follows:
, where Rap is the incidence or mortality rate for the age group, a is the age at diagnosis in the calendar period, p is the diagnosis year, and Aa, Pp, and Cc are the non-linear composition of the age group (a), period (p), and cohort (c), respectively.
Next, the incidence-prevalence-mortality (IPM) model was used to project the future prevalence of breast cancer [16], the DisMod II program was used to estimate the average age of onset and duration of disability using the projected future incidence mortality and prevalence [17], and, finally, the GBD method was used to calculate DALYs [18]. Average annual percent changes (AAPC) for YLL, years lived with disability (YLD), and DALYs were estimated using the Joinpoint analysis. The population attributable risk (PAR) was calculated using the meta-analyzed relative risks (RRs) and prevalence of risk factors (p). Levin’s formula was used to calculate the PAR for multiple categories [19], as follows:
DALYs were calculated as the sum of the YLL and YLD. The number of breast cancer deaths and standard life expectancy at the age of death were used for estimating the YLL. The number of incident breast cancer cases, disability weight (DW), and average duration of breast cancer were used for estimating the YLD. To project the future YLL and YLD, we applied the Nordpred APC model, IPM model, and DisMod II program. The data source and summarized measures are shown in Table 1 [7,20-23]. The GBD formula to estimate the YLL, which was improved by using the Nordpred APC model, in this study was as follows:
, where a is the average age at onset for each age cohort; the discount rate (r) is 0.03; the age-weighting constants are β=0.04, K=1.00, and C=0.1658; M is mortalities; and L is the standard life expectancy at the age of death.
In the current study, we modified the following GBD formula to estimate the YLD with the Nordpred APC and IPM models:
, where I is the incidences; the DW is 0.37; a is the average age at onset for each age cohort; r is 0.03; the age-weighting constants are β=0.04, K=1.00, and C=0.1658; and L is the expected duration of disability.
Of the breast cancer risk factors, we selected modifiable and rapidly changing risk factors (i.e., age at first childbirth, total breastfeeding period, obesity, alcohol drinking, OC use, and HRT use). For PAR calculations, the RRs were determined from the National Cancer Institute report [13,24]; the prevalence rates of obesity (body mass index ≥ 25 kg/m2) and alcohol consumption (monthly drinking) were determined from Statistics Korea [25]; the total breastfeeding period, OC use, and HRT use were obtained from the Korea National Health and Nutrition Examination Survey [26] and Korea Institute for Health and Social Affairs [27]; and the age at first childbirth was determined from the Micro-data Integrated Service [28]. In this study, we assumed a latency period of about 15 years between the exposure of a risk factor and breast cancer development, except for OC and HRT use [24]. Specifically, OC and HRT use are associated with an increased risk of breast cancer, but the risk declines rapidly after cessation of OC and HRT [29]. Therefore, we did not assume a latency period for OC and HRT use, and we assumed that the annual increasing rate for the past (1998-2000) and future prevalence rates were 0% for OC use and 2% for HRT use, considering each trend of OC and HRT use during 2001-2015 [26].
The projected age-specific incidence rates of breast cancer per 100,000 women from 2000 to 2030 are shown in Fig. 1. According to the recent trends of age-specific breast cancer incidence rates, the incidence rates peak sharply in women aged 45-49 years, and the future age-specific incidence rate will peak in women of older age groups. The future trend in the Korean breast cancer age-specific incidence curve is similar to the current Western breast cancer curve.
The projected age-specific mortality rates of breast cancer per 100,000 women from 2000 to 2030 are shown in Fig. 2. When the age-specific mortality rates were projected, the peak age was observed in women aged ≥ 85 years, and the future age-specific mortality rates are expected to increase.
The RRs of breast cancer risk factors and changes in the prevalence rates of these risk factors according to women’s lifestyle changes between 1998 and 2015 are shown in Table 2. Reproductive and lifestyle risk factors of breast cancer have changed considerably in Korean women because of the rapid modernization and economic growth in South Korea. The percentage of women who experienced their first childbirth at a delayed age (i.e., ≥ 31 years old) has increased from 9.5% in 1998 to 48.6% in 2015. However, the percentage of women who breastfed increased from 10.2% to 36.2%. The percentages of obesity and alcohol consumption were also increased during the period (obesity, from 25.9% to 28.8%; alcohol consumption, from 32.7% to 46.5%). In terms of OC and HRT use, the estimated percentages of OC and HRT use are 15.7% and 3.4%, respectively, in 2030, considering the annual increasing rate of OC and HRT use during 2001-2015.
The projected trends for the DALYs, YLL, and YLD per 100,000 Korean women with breast cancer from 1999 to 2030 are shown in 5-year increments in Fig. 3. There was a slight increase in the estimated trend in the YLL per 100,000 women from 1999 to 2030 (from 105 to 165 person-years; AAPC, 1.37%) (S1 Table). However, the YLD, which was less than half of the YLL in 1999 (50 YLD per 100,000 personyears) and higher than the YLL in 2007 (136 YLL per 100,000 person-years;155 YLD per 100,000 person-years), is rapidly increasing, and this increasing trend will continue to 2030 (423 YLD per 100,000 person-years; AAPC, 5.38%) based on our projection (S2 Fig.). The trend for DALYs was similar to the increasing trend for the YLD (155 DALYs per 100,000 person-years in 1999, 395 DALYs per 100,000 person-years in 2013, and 588 DALYs per 100,000 person-years in 2030; AAPC, 3.84%). Furthermore, the percentage of breast cancer burden attributable to lifestyle changes was predicted to increase from 34.2% to 44.3% (135 DALYs per 100,000 person-years in 2013, 260 DALYs per 100,000 person-years in 2030).
The YLD was estimated using the average age of onset and breast cancer duration. The estimated average age of onset and average duration of disability using the projected future incidence mortality and prevalence are shown in S3 Table.
The projected burden (DALYs) of breast cancer per 100,000 women that can be attributable to lifestyle changes is shown in Fig. 4. The percentage of women experiencing their first childbirth at ≥ 31 years increased dramatically by about 5 times during 1998-2015 (from 0.1% to 0.49%). Postponing the first childbirth during the period could add an extra 48 person-years to the future burden of breast cancer attributable to the age at first childbirth per 100,000 women in 2030, which is about 2.3 times the burden of breast cancer in 2013 (37 DALYs per 100,000 person-years in 2013, 85 DALYs per 100,000 person-years in 2030). The increase (~3%) in obesity during 1998-2015 could add 24 person-years of additional burden of breast cancer attributable to obesity in 2030, which is approximately 1.6 times the burden of breast cancer in 2013 (37 DALYs per 100,000 person-years in 2013, 61 DALYs per 100,000 person-years in 2030). The increasing trend of alcohol consumption during 1998 to 2013 could be attributable for 20 person-years of future burden of breast cancer in 2030, which is about 2 times the burden of breast cancer in 2013 (19 DALYs per 100,000 person-years in 2013, 39 DALYs per 100,000 person-years in 2030). The increasing trend in breastfeeding for less than 6 months (as compared to ≥ 7 months) during 1998-2015 was predicted to add 24 person-years of future burden of breast cancer in 2030 (22 DALYs per 100,000 person-years in 2013, 46 DALYs per 100,000 person-years in 2030). Finally, we expect that the trends for OC and HRT use during 2001-2015 will continue in the next decade. OC and HRT use are predicted to add 9 and 1 person-year(s), respectively, to the burden of breast cancer in 2030.
The burden of breast cancer has become heavier, owing to the considerably increased incidence and survival rate of breast cancer. These trends may reflect changes in lifestyle, age structure, and medical technology in the last decade [3,30].
In this study, we estimated and projected the trends in the DALYs, YLL, and YLD for breast cancer in Korean women during 1999-2030, and the burden of breast cancer attributable to lifestyle changes during 2013-2015. We considered several modifiable reproductive and lifestyle-related risk factors of breast cancer. In fact, the majority of breast cancer risk factors are modifiable; these include age at first childbirth, total breastfeeding duration, obesity, alcohol consumption, OC use, and HRT use. In this study, in 2030, the burden of breast cancer attributable to advanced age at first childbirth was predicted to be the highest, followed by the burden attributable to obesity.
Korea's fertility rate has started to experience a lower birth rate than that of Organization for Economic Co-operation and Development (OECD) average since 1984, reaching 1.17; the lowest among OECD countries in 2016, despite the governmental full support to increase it. Furthermore, fertile Korean female population's delaying age of its first childbirth is on progress [31]. Therefore, the burden of breast cancer attributable to late age at first childbirth is projected to keep worsening. Although > $70 billion have been spent to raise the low birthrate over the last decade, Korea has maintained a low-fertility rate during the past 16 years [32]. Furthermore, the proportion of older mothers (> 35 years) has more than doubled to 1 in 4. On the other hand, the proportion of mothers in their early 20s has considerably decreased from 40.5% in 1984 to 5.2% in 2010 and that of mothers in their late 20s also decreased from 54.6% in 1970 to 31.4% in 2010 [33,34]. These trends might be caused by a high unemployment rate and the older age at marriage [35]. Therefore, solutions to address unemployment and residence problems for young people should be priorities of the Korean government.
There are many national breast cancer prevention support programs, including primary, secondary, and tertiary preventions, such as financial support for cancer patients, breast cancer self-check campaigns, and quality care for cancer patients. Moreover, the Korean government offers breast screening services through the National Cancer Screening Program. However, there is a lack of effort to improve public awareness about the magnitude of modifiable breast cancer risk factors and the effect of cancer prevention. In recent studies, it has been reported that lifestyle changes can prevent 25%-30% of breast cancer cases [36]. However, most people consider that the majority of breast cancer cases are caused by genetic factors and are beyond individual control [12]. Therefore, education campaigns about lifestyle changes (e.g., moderate alcohol consumption, physical activity, and/or weight control) and their effects are important and necessary for reducing the burden of breast cancer.
There are several limitations in this study. First, we adopted RR estimations, as reported by the National Cancer Institute. These RRs are representative estimations, which were meta-analyzed using nationwide representative sample data. However, as the meta-analysis estimations may include heterogeneity between studies, the RR estimations should be interpreted with caution. Second, accurate estimations of the past (1998-2000) and future prevalence of OC and HRT use were not available. Therefore, we predicted that the annual increasing rate for the past (1998-2000) and future prevalence of OC and HRT were 0% and 2%, respectively, assuming that the trends for OC and HRT use were maintained during 2001-2015. Third, we were unable to consider all possible risk factors associated with breast cancer due to the unavailability of past prevalence data, such as percentages of each category according to physical activity, the number of children, and age at menarche. Fourth, although we used nationwide cancer data, including both cancer incidence and mortality, the projected results should be interpreted with caution as we did not analyze any individual data in this study. However, using national data, including nationwide cancer incidence and mortality data, ensures the external validity and permits comparisons with other countries.
Despite these limitations, this study also has several strengths. First, we measured the burden of disease as DALYs, which indicate the lost time of healthy life, and we did not rely on only breast cancer incidence or mortality as the study outcome. Second, the breast cancer-specific incidence and mortality rates were projected using the APC model in addition to the simplified GBD equation, with a linear relationship, leading to more accurate estimations considering the age, period, and birth cohort effect. Third, we used nationwide cancer data in Korea, classified by the cancer type and age, for breast cancer specific-incidence and mortality, and nationwide community-based sample data for risk factor prevalence. Using these nationwide data increases the external validity of this study and makes the results more representative. Fourth, most researchers provide estimations of either PAR or the burden of breast cancer, but not both. However, we projected the burden of breast cancer attributable to modifiable risk factors. Furthermore, we estimated the proportions of risk factors from 1998 to 2015, considering a latency period of 15 years between the exposure of the risk factor and breast cancer development.
The dramatically increased burden of breast cancer is an important issue. Moreover, changes in reproductive and lifestyle factors (i.e., advanced maternal age at first childbirth, alcohol consumption, and obesity) will worsen the future burden of breast cancer. The current findings suggest that successful control of reproductive/lifestyle factors could reduce 34.2% to 44.3% of the burden of breast cancer in 2030. Policy makers should concentrate on lifestyle modifications to reduce the long-term future burden of breast cancer in Korea. Lifestyle modifications that encourage pregnancy before advanced maternal age and breastfeeding for ≥ 7 months, and that reduce obesity and alcohol consumption could reduce the risks of breast cancer.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (http://www.e-crt.org).
References
1. Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016; 48:436–50.

2. National Cancer Information Center. Survival rates of all cancers. Goyang: National Cancer Center;2016.
3. Park SK, Kim Y, Kang D, Jung EJ, Yoo KY. Risk factors and control strategies for the rapidly rising rate of breast cancer in Korea. J Breast Cancer. 2011; 14:79–87.

4. Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet. 2017; 389:1323–35.

5. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014; 74:2913–21.

6. Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997; 349:1498–504.

7. World Health Organization. World Health Organization global burden of disease. Geneva: World Health Organization;2007.
8. Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH. Prediction of cancer incidence and mortality in Korea, 2017. Cancer Res Treat. 2017; 49:306–12.

9. Jang SI, Nam JM, Choi J, Park EC. Disease management index of potential years of life lost as a tool for setting priorities in national disease control using OECD health data. Health Policy. 2014; 115:92–9.

10. Kunnavil R, Thirthahalli C, Nooyi SC, Somanna SN, Murthy NS. Estimation of burden of female breast cancer in India for the year 2016, 2021 and 2026 using disability adjusted life years. Int J Community Med Public Health. 2016; 3:1135–40.
11. Asadzadeh Vostakolaei F, Broeders MJ, Mousavi SM, Kiemeney LA, Verbeek AL. The effect of demographic and lifestyle changes on the burden of breast cancer in Iranian women: a projection to 2030. Breast. 2013; 22:277–81.
12. Harvie M, Howell A, Evans DG. Can diet and lifestyle prevent breast cancer: what is the evidence? Am Soc Clin Oncol Educ Book. 2015; e66–e73.

13. Park B, Park S, Shin HR, Shin A, Yeo Y, Choi JY, et al. Population attributable risks of modifiable reproductive factors for breast and ovarian cancers in Korea. BMC Cancer. 2016; 16:5.

14. Hayes J, Richardson A, Frampton C. Population attributable risks for modifiable lifestyle factors and breast cancer in New Zealand women. Intern Med J. 2013; 43:1198–204.

15. Moller B, Fekjaer H, Hakulinen T, Sigvaldason H, Storm HH, Talback M, et al. Prediction of cancer incidence in the Nordic countries: empirical comparison of different approaches. Stat Med. 2003; 22:2751–66.
16. Kruijshaar ME, Barendregt JJ, Van De Poll-Franse LV. Estimating the prevalence of breast cancer using a disease model: data problems and trends. Popul Health Metr. 2003; 1:5.

17. Barendregt JJ, Van Oortmarssen GJ, Vos T, Murray CJ. A generic model for the assessment of disease epidemiology: the computational basis of DisMod II. Popul Health Metr. 2003; 1:4.

18. World Health Organization. The global burden of disease concept. Geneva: World Health Organization;2016.
19. Hanley JA. A heuristic approach to the formulas for population attributable fraction. J Epidemiol Community Health. 2001; 55:508–14.

20. Statistics Korea. Korean statistical information service: population (estimated population, life table), welfare (causes of death, cancer registry). Daejeon: Statistics Korea;2015.
21. National Cancer Institute. Cancer statistics. Goyang: National Cancer Center;2013.
22. Park E, Park J. The analysis and reduction strategies of cancer burden in Korea. Seoul: Korean Foundation for Cancer Research;2012.
23. Barendregt JJ, Van Oortmarssen GJ, Vos T, Murray CJ. A generic model for the assessment of disease epidemiology: the computational basis of DisMod II. Popul Health Metr. 2003; 1:4.

24. Park SH. Estimation of population attributable fraction for cancer risk factors and prediction of cancer incidence and mortality rates in Korea. Goyang: National Cancer Center;2009.
25. Statistics Korea. Social survey [Internet]. Daejeon: Statistics Korea;2015. [cited 2017 Jul 21]. Available from: http://kostat.go.kr/.
26. Korea Centers for Disease Control and Prevention. Korea National Health and Nutrition Examination Survey, 2001-2015 [Internet]. Cheongju: Korea Centers for Disease Control and Prevention;2017. [cited 2017 Jul 21]. Available from: https://knhanes.cdc.go.kr/knhanes/eng/index.do/.
27. Park IH, Hwang NM. The analysis of breast feeding status and breast feeding supporting policy. Sejong: Korea Institute for Health and Social Affairs;1994.
28. Microdata Integrated Service. Birth data 1998-2015. [Internet]. Daejeon: Statistics Korea;2015. [cited 2017 Jul 21]. Available from: https://mdis.kostat.go.kr/.
29. Li L, Ji J, Wang JB, Niyazi M, Qiao YL, Boffetta P. Attributable causes of breast cancer and ovarian cancer in china: reproductive factors, oral contraceptives and hormone replacement therapy. Chin J Cancer Res. 2012; 24:9–17.

30. Park JH, Lee KS, Choi KS. Burden of cancer in Korea during 2000-2020. Cancer Epidemiol. 2013; 37:353–9.

31. Statistics Korea. Total fertility rate [Internet]. Daejeon: Statistics Korea;2016. [cited 2017 Jul 21]. Available from: https://mdis.kostat.go.kr/.
32. Ma L. Female labour force participation and second birth rates in South Korea. J Popul Res. 2016; 33:173–95.

33. Statistics Korea. Birth statistics [Internet]. Daejeon: Statistics Korea;2016. [cited 2017 Jul 21]. Available from: https://mdis.kostat.go.kr/.
34. Lim JW. The changing trends in live birth statistics in Korea, 1970 to 2010. Korean J Pediatr. 2011; 54:429–35.

35. Kim MH. The trend of international population aging. Vol. 2. Seoul: Korea Insurance Research Institute;2016. p. 21–3.
Fig. 1.
Projected age-specific incidence rates of breast cancer per 100,000 women in South Korea from 2000 to 2030.
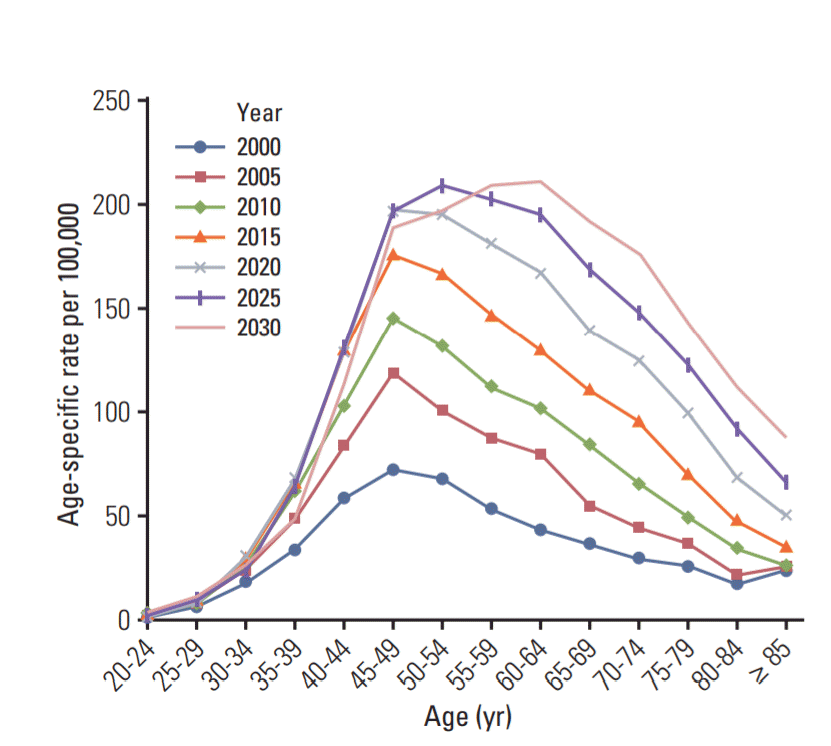
Fig. 2.
Projected age-specific mortality rates of breast cancer per 100,000 women in South Korea from 2000 to 2030.
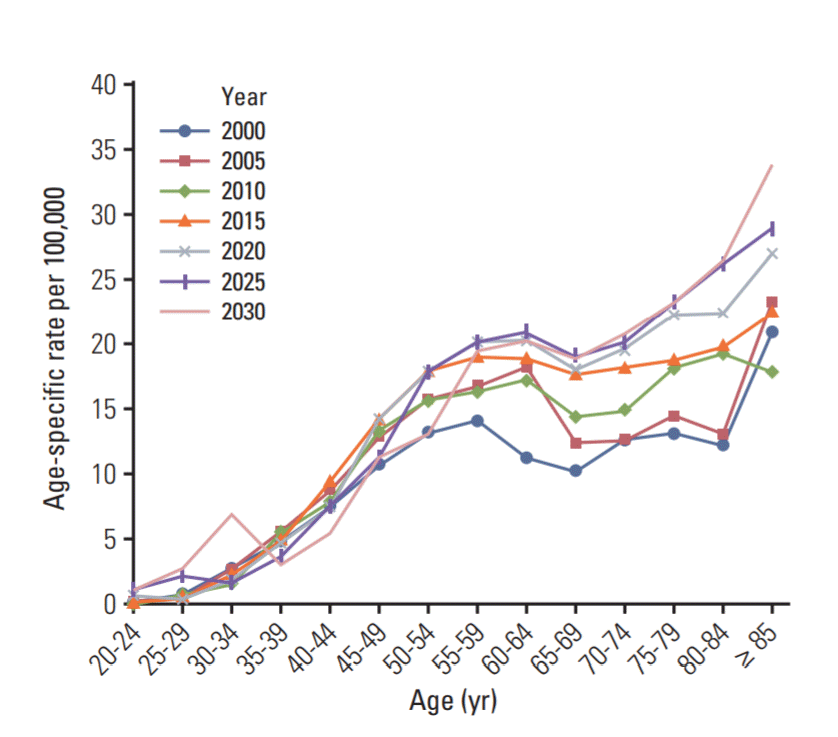
Fig. 3.
Projected disability-adjusted life years (DALYs), years of life lost (YLL), and years lived with disability (YLD) trends of breast cancer in Korean women from 1999 to 2030.
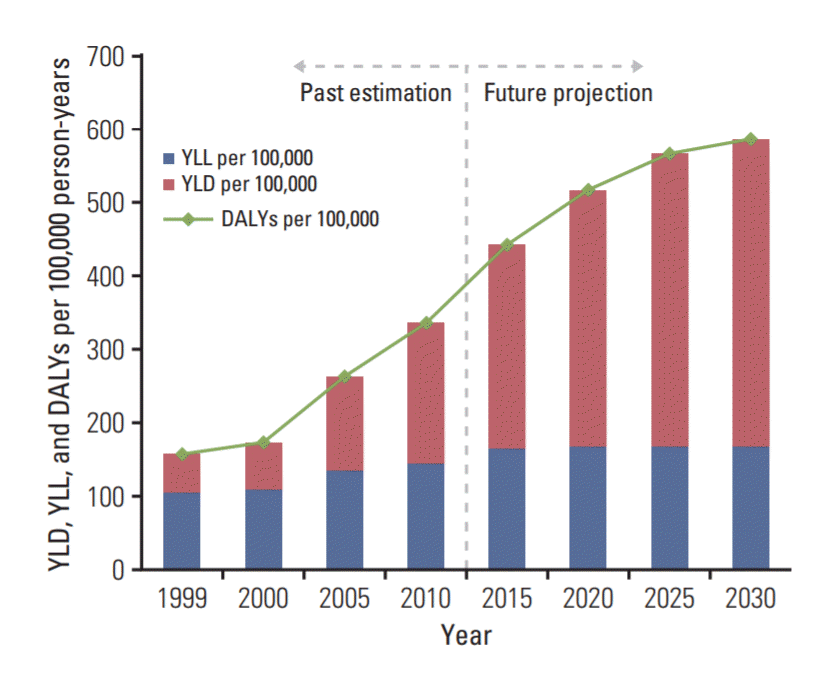
Fig. 4.
Projected burden of breast cancer attributable to lifestyle changes (disability-adjusted life years [DALYs] per 100,000 women).
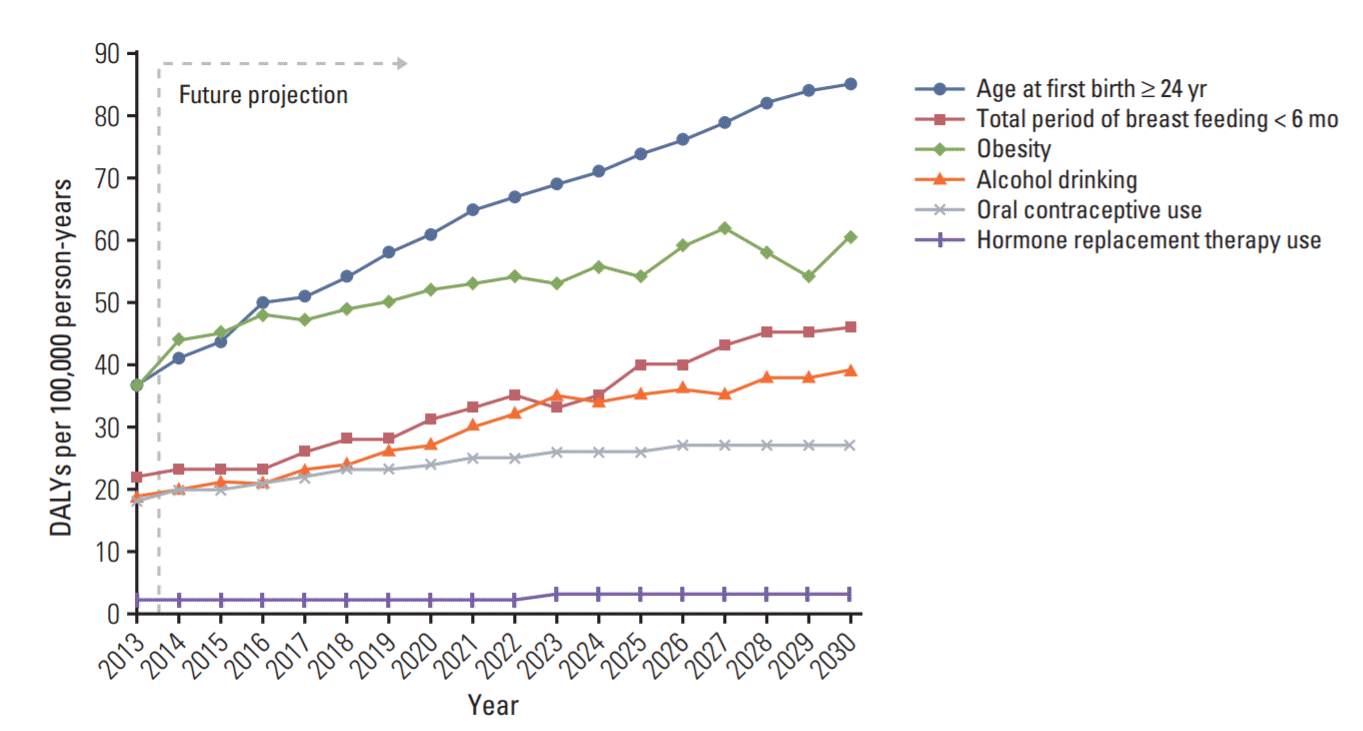
Table 1.
Data sources and Global Burden of Disease Study Group formulae
| Measure | Study |
|---|---|
| Years of life lost (YLL) | |
| YLL=M×{[KCera/(r+β)2][e–(r+β)(L+a)[–(r+β)(L+a)–1]–e–(r+β)a[–(r+β)a–1]]+[(1–K)/r](1–e–rL)} | |
| Average age at onset for each age cohort (a) | Average age |
| Discount rate (r=0.03) | [7] |
| Age-weighting constants (β=0.04, K=1.00, C=0.1658) | [7] |
| Mortalities (M) | [20] |
| Standard life expectancy at the age of death (L) | [20] |
| Years lived with disability (YLD) | |
| YLD=I×DW×{[KCera/(r+β)2][e–(r+β)(L+a)[–(r+β)(L+a)–1]–e–(r+β)a[–(r+β)a–1]]+[(1–K)/r](1–e–rL)} | |
| Incidences (I) | [21] |
| Disability weights (DW=0.37) | [22] |
| Average age at onset for each age cohort (a) | [23] |
| Discount rate (r=0.03) | [7] |
| Age-weighting constants (β=0.04, K=1.00, C=0.1658) | [7] |
| Expected duration of disability (L) | [23] |
Table 2.
Relative risks and changes in the prevalence (%) of breast cancer risk factors according to lifestyle changes from 1998 to 2015
This study assumed a latency period of about 15 years between the exposure of a risk factor and breast cancer development, except for oral contraceptive (OC) and hormone replacement therapy (HRT) use. We assumed the annual increasing rates for the future prevalence rates of OC and HRT use to be 0% and 2%, respectively, considering the trend for OC and HRT use during 2001-2015. CI, confidence interval; BMI, body mass index.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download