Introduction
Materials and Methods
1. Study population
2. Case and control selection
3. Variables
4. Statistical analysis
5. Ethical statement
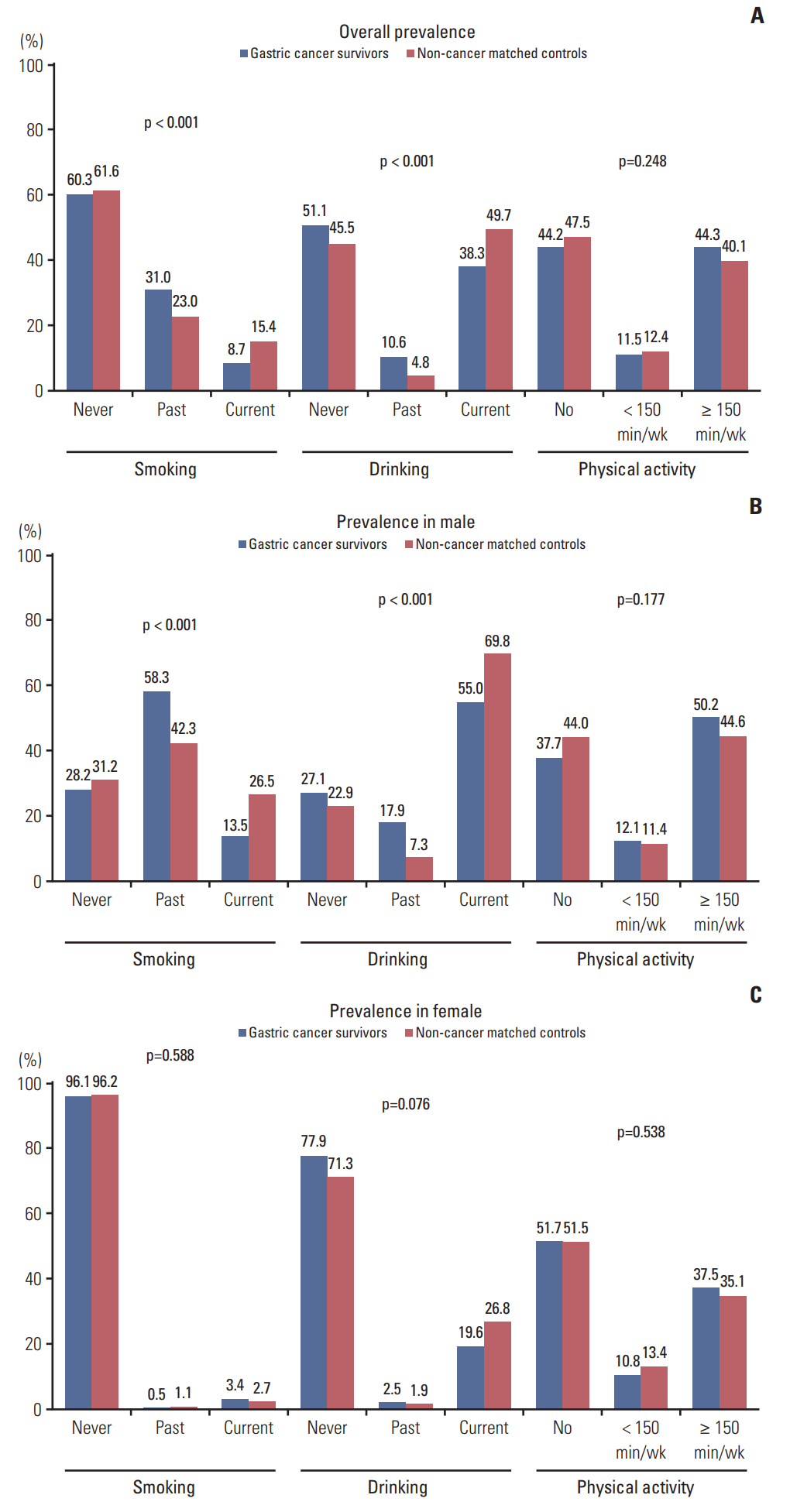
Results
Table 1.
| Characteristica) | Survivors (n=437) | Non-cancer controls (n=4,370) | p-valueb) |
|---|---|---|---|
| Age, mean (95% CI) | 58.6 (57.9-59.4) | 58.5 (58.2-58.7) | 0.650 |
| Age (yr) | |||
| < 50 | 64 (14.7) | 640 (14.7) | 1.000 |
| 50-54 | 75 (17.2) | 750 (17.2) | |
| 55-59 | 75 (17.2) | 750 (17.2) | |
| ≥ 60 | 223 (51.0) | 2,230 (51.0) | |
| Sex | |||
| Male | 232 (53.1) | 2,320 (53.1) | 1.000 |
| Female | 205 (46.9) | 2,050 (47.0) | |
| Marital status | |||
| Married, cohabitation | 399 (91.7) | 3,856 (88.7) | 0.054 |
| Divorced, widow, unmarried | 36 (8.3) | 492 (11.3) | |
| Educational attainment | |||
| ≤ High school | 323 (74.8) | 3,178 (73.5) | 0.562 |
| > College | 109 (25.2) | 1,147 (26.5) | |
| Household income | |||
| < $2,000 | 186 (48.1) | 1,815 (46.8) | 0.629 |
| ≥ $2,000 | 201 (52.0) | 2,065 (53.2) | |
| Employment state | |||
| Currently unemployed | 230 (53.1) | 2,037 (47.6) | 0.029 |
| Currently employed | 203 (46.9) | 2,242 (52.4) | |
| Self-rated health status | |||
| Healthy | 157 (36.3) | 1,797 (41.5) | 0.034 |
| Normal-unhealthy | 276 (63.7) | 2,532 (58.5) | |
| Co-morbid chronic diseasec) | |||
| Yes | 226 (51.7) | 2,308 (52.8) | 0.661 |
| No | 211 (48.3) | 2,062 (47.2) | |
| Family history of cancer (any type) | |||
| Yes | 178 (41.1) | 1,079 (24.8) | < 0.001 |
| No | 255 (58.9) | 3,275 (75.2) | |
| Years since diagnosis | |||
| 2-5 | 161 (36.8) | - | < 0.001 |
| ≥ 6 | 271 (62.0) | - | |
| Current smoking status | |||
| Yes | 38 (8.7) | 668 (15.4) | 0.001 |
| No | 397 (91.3) | 3,685 (84.7) | |
| Current drinking status | |||
| Yes | 166 (38.3) | 2,162 (49.7) | < 0.001 |
| No | 267 (61.7) | 2,192 (50.3) | |
| Physical activity | |||
| < 150 min/wk | 242 (55.8) | 2,521 (59.9) | 0.095 |
| ≥ 150 min/wk | 192 (44.2) | 1,688 (40.1) | |
| BMI | |||
| < 23 | 278 (63.6) | 1,535 (35.2) | < 0.001 |
| ≥ 23 | 159 (36.4) | 2,826 (64.8) |
Values are presented as number (%) unless otherwise indicated. CI, confidence intervals; BMI, body mass index.
a) Missing values (No. of survivors, No. of non-cancer controls): marital status (2, 22), educational attainment (5, 45), household income (50, 490), employment state (4, 91), self-rated health status (4, 41), family history of cancer (4, 16), current smoking status (2, 17), current drinking status (4, 16), physical activity (3, 161), BMI (0, 9),
c) Any of following diseases were classified as chronic disease: hypertension, diabetes, dyslipidemia, stroke, transient ischemic attacks, angina or myocardiac infarction, colon polyp, fatty liver, chronic liver disease or liver cirrhosis, gallbladder stone or cholecystitis, thyroid disease, arthritis, and osteoporosis.
Table 2.
| Characteristic |
Current smoking |
Current drinking |
Physical inactivitya) |
|||
|---|---|---|---|---|---|---|
| Odds ratio (95% confidence interval) | p-value | Odds ratio (95% confidence interval) | p-value | Odds ratio (95% confidence interval) | p-value | |
| Age, 1-year increment | 0.93 (0.88-0.99) | 0.024 | 1.00 (0.97-1.03) | 1.000 | 1.00 (0.97-1.04) | 0.846 |
| Sex | ||||||
| Male | 1 | 1 | 1 | |||
| Female | 0.16 (0.05-0.46) | 0.001 | 0.23 (0.14-0.40) | < 0.001 | 2.12 (1.26-3.57) | 0.005 |
| Marital status | ||||||
| Divorced, widow, unmarried | 1 | 1 | 1 | |||
| Married, cohabitation | 0.41 (0.10-1.77) | 0.233 | 0.81 (0.34-1.94) | 0.633 | 0.96 (0.43-2.13) | 0.921 |
| Educational attainment | ||||||
| ≤ High school | 1 | 1 | 1 | |||
| > College | 0.39 (0.14-1.09) | 0.073 | 0.85 (0.50-1.44) | 0.543 | 0.67 (0.41-1.10) | 0.115 |
| Household income | ||||||
| < $2,000 | 1 | 1 | 1 | |||
| ≥ $2,000 | 0.66 (0.26-1.69) | 0.388 | 1.74 (1.03-2.94) | 0.040 | 1.00 (0.62-1.63) | 0.999 |
| Employment state | ||||||
| Currently unemployed | 1 | 1 | 1 | |||
| Currently employed | 1.86 (0.76-4.56) | 0.175 | 1.36 (0.84-2.21) | 0.208 | 1.97 (1.24-3.12) | 0.004 |
| Self-rated health status | ||||||
| Healthy | 1 | 1 | 1 | |||
| Normal-unhealthy | 1.37 (0.61-3.08) | 0.441 | 0.84 (0.53-1.35) | 0.475 | 1.57 (1.02-2.43) | 0.042 |
| Family history of cancera) | ||||||
| No | 1 | 1 | 1 | |||
| Yes | 1.40 (0.66-3.00) | 0.382 | 0.94 (0.60-1.46) | 0.775 | 1.04 (0.69-1.57) | 0.851 |
| Co-morbid chronic diseaseb) | ||||||
| No | 1 | 1 | 1 | |||
| Yes | 0.56 (0.25-1.27) | 0.165 | 0.97 (0.61-1.53) | 0.889 | 0.65 (0.42-0.99) | 0.048 |
| Years since diagnosis | ||||||
| ≤ 5 | 1 | 1 | 1 | |||
| > 6 | 3.17 (1.27-7.90) | 0.013 | 1.44 (0.90-2.28) | 0.127 | 1.31 (0.86-2.01) | 0.213 |
| Current smoking | ||||||
| No | - | 1 | 1 | |||
| Yes | - | - | 2.43 (1.11-5.33) | 0.027 | 1.10 (0.52-2.33) | 0.803 |
| Current drinking | ||||||
| No | 1 | - | 1 | |||
| Yes | 2.48 (1.13-5.45) | 0.024 | - | - | 1.13 (0.72-1.78) | 0.594 |
| Physical activity | ||||||
| < 150 min/wk | 1 | 1 | - | |||
| ≥ 150 min/wk | 0.98 (0.45-2.16) | 0.960 | 0.89 (0.56-1.40) | 0.607 | - | - |
| Body mass index | ||||||
| < 23 kg/m2 | 1 | 1 | 1 | |||
| ≥ 23 kg/m2 | 0.67 (0.30-1.54) | 0.351 | 1.39 (0.88-2.19) | 0.155 | 0.77 (0.50-1.17) | 0.216 |
b) Any of following diseases were classified as chronic disease: hypertension, diabetes, dyslipidemia, stroke, transient ischemic attacks, angina or myocardial infarction, colon polyp, fatty liver, chronic liver disease or liver cirrhosis, gallbladder stone or cholecystitis, thyroid disease, arthritis, and osteoporosis.




 Citation
Citation Print
Print


 XML Download
XML Download