Introduction
The formation of cancer may be related to environmental carcinogens, genes and immunity of the subject. The formation of skin cancer is also related to these factors. A person with a cancer might represent that there are abnormalities in environment and genes of this subject; therefore, the subject has an increased opportunity of having a second primary cancer involving other organs. Such an increased risk of second primary cancer has been observed in people affected by gastrointestinal cancer and head and neck cancer [
1,
2]. Also, previous western studies have found Caucasians with skin cancer, either melanoma or non-melanoma skin cancer (NMSC), have an elevated risk of second primary cancer [
3,
4]. On the other hand, ultraviolet radiation, the main environmental cause of skin cancer, is known to promote the synthesis of vitamin D which in turn has been associated with a decrease in the risk of prostate, breast, and colorectal cancer [
5]. A Dutch study identified a lowered risk of colorectal cancer in people with skin cancer [
6], but a Swiss study found the risk of prostate, breast and colorectal cancer all increased in those with skin cancer [
5].
Previous western studies identified men and women differed in the risk for various subsequent cancers. A U.S. study found men with NMSC had an increased risk of subsequent melanoma, while women with NMSC had an increased risk of subsequent breast and lung cancer as well as melanoma [
4]. A Norwegian study showed men with melanoma were prone to have subsequent prostate and thyroid cancers, whereas women with melanoma were more likely to have subsequent cancers involving the breast and central nervous system [
3].
A study found an elevated risk of second primary cancer in Asians with melanoma [
7]. However, to date there have been no similar studies examining whether Asians with NMSC have an increased risk of second primary cancer involving other organs.
The objective of this retrospective cohort study was to assess whether people diagnosed with a NMSC would have an increased risk of second primary cancer involving other organs in a Taiwanese setting. It is expected that the results of this study will be a useful reference for health education and preventive medicine interventions for people diagnosed with NMSC.
Go to :

Results
The characteristics and potential confounders of the study subjects are listed in
Table 1. We identified 505 people with NMSC and 2,020 matched controls without NMSC. No significant differences in the age and gender distribution, urbanization as well as the length of follow up were found between the NMSC and control groups. A higher score of CCI was found with NMSC patient compared to people without NMSC, being 4.7±3.2 and 3.3±2.7, respectively.
Table 1.
Characteristics and potential confounders of the study subjects
|
Control (n=2,020) |
Non-melanoma skin cancer group (n=505) |
p-value |
|
Sex
|
|
|
|
|
Women |
1,024 (80.0) |
256 (20.0) |
1.000 |
|
Men |
996 (80.0) |
249 (20.0) |
|
|
Age (yr)
|
67.2±15.2 |
67.2±15.4 |
0.96 |
|
Urbanization
|
|
|
|
|
City |
893 (44.2) |
223 (44.2) |
1.000 |
|
Suburbs |
747 (37.0) |
187 (37.0) |
|
|
Village |
380 (18.8) |
95 (18.8) |
|
|
Charlson Comorbidity Index
|
3.3±2.7 |
4.7±3.2 |
< 0.001 |
|
Length of follow-up (yr)
|
6.0±3.0 |
5.7±3.1 |
0.05 |

As show in
Table 2, the NMSC group had a twice higher incidence of second primary cancer than the control group. The Kaplan-Meier curves of cumulative incidence for second primary cancer are shown in
Fig. 2. The NMSC group had a significant higher probability of developing second primary cancer than controls (p=0.024) (
Fig. 2A). When compared to the control group, the NMSC group had an increased risk of second primary cancer (crude, 1.87; 95% confidence interval [CI], 1.37 to 2.55). After controlling for potential confounders including age, sex, urbanization, and CCI, the adjusted HR was 1.43 (95% CI, 1.05 to 1.96).
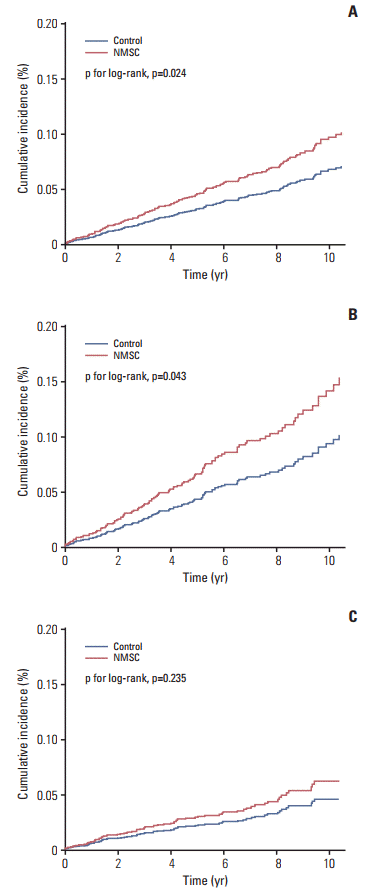 | Fig. 2.Cumulative incidence curves of second primary cancer in people with non-melanoma skin cancer (NMSC). (A) All subjects. (B) Men. (C) Women. 
|
Table 2.
Risk of second primary cancer in people with non-melanoma skin cancer
|
No. |
No. of incident second primary cancer |
No. of person-years |
Incidence (per 102 person-year) |
Adjusted HR (95% CI)a)
|
|
Total
|
2,525 |
188 |
14,931.71 |
1.26 (1.09-1.45) |
- |
|
Control |
2,020 |
130 |
12,068.08 |
1.08 (0.90-1.27) |
1.00 (reference) |
|
NMSC |
505 |
58 |
2,863.63 |
2.03 (1.55-2.59) |
1.43 (1.05-1.96) |
|
All men
|
1,245 |
116 |
7,345.79 |
1.58 (1.31-1.88) |
- |
|
Control |
1,129 |
80 |
5,972.34 |
1.34 (1.07-1.65) |
1.00 (reference) |
|
NMSC |
116 |
36 |
1,373.45 |
2.62 (1.86-3.57) |
1.51 (1.01-2.25) |
|
All women
|
1,280 |
72 |
7,585.93 |
0.95 (0.75-1.19) |
- |
|
Control |
1,208 |
50 |
6,095.75 |
0.82 (0.61-1.07) |
1.00 (reference) |
|
NMSC |
72 |
22 |
1,490.18 |
1.48 (0.94-2.18) |
1.35 (0.82-2.24) |

When stratified by sex, both men and women with NMSC had a nearly twice higher incidence of second primary cancer than their respective controls. As shown in
Fig. 2B and
C, men with NMSC had a higher probability of second primary cancer than controls (p=0.043), but women did not (p=0.235). After adjusted for potential confounders, men with NMSC had a significantly higher risk of second primary cancer than controls (adjusted HR, 1.51; 95% CI, 1.01 to 2.25) but women with NMSC did not (adjusted HR, 1.35; 95% CI, 0.82 to 2.24).
In the analysis according to involved organ of second primary cancer, men with NMSC had a significant higher risk of second primary cancer involving the lip, oral cavity, pharynx (adjusted HR, 2.99; 95% CI, 1.00 to 9.10) and the genitourinary organs (adjusted HR, 3.51; 95% CI, 1.21 to 10.17) (
Table 3). The Kaplan-Meier curves of cumulative incidence for second primary cancer involving the lip, oral cavity, pharynx and the genitourinary organs in men are shown in
Fig. 3A and
B. Compared to the control group, men with NMSC had a significantly higher probability of second primary cancer affecting the lip, oral cavity, pharynx (p=0.050), and the genitourinary organs (p=0.021).
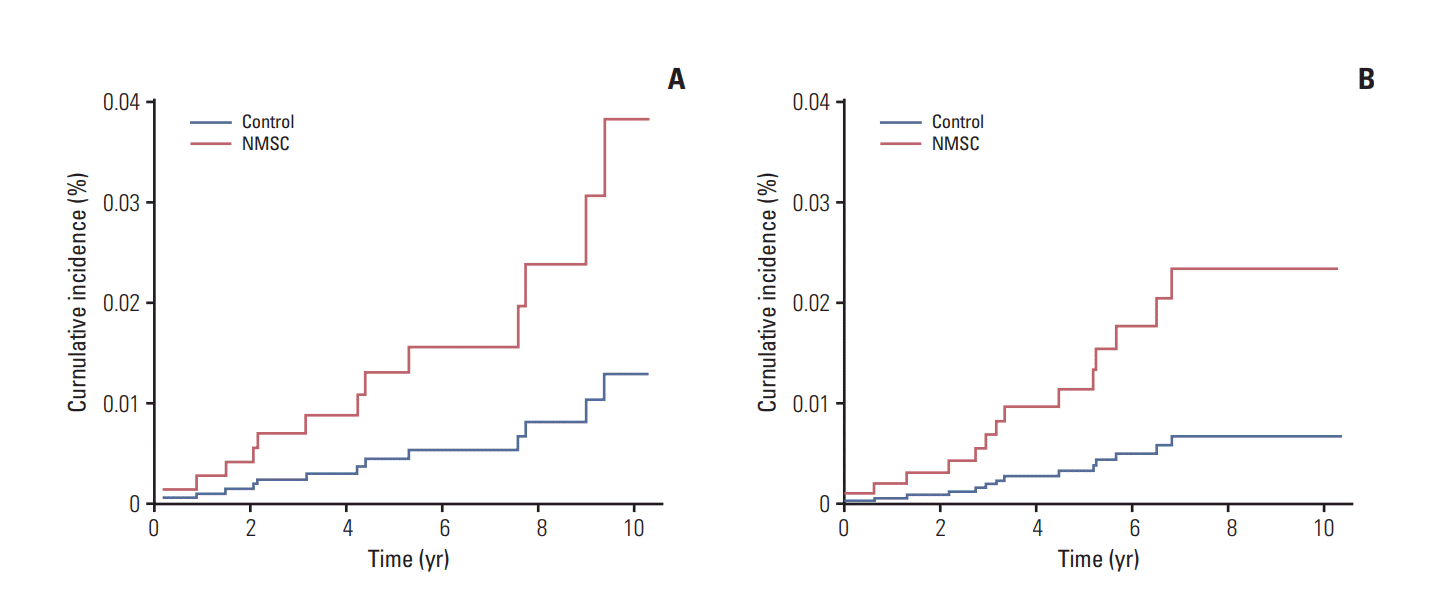 | Fig. 3.Cumulative incidence curves of second primary cancer involving the lip, oral cavity, pharynx (A), and the genitourinary organs (B) in men. NMSC, non-melanoma skin cancer. 
|
Table 3.
Stratified analysis based on sex
|
Second primary cancer |
Men (n=1,245)
|
Women (n=1,280)
|
No. (%)
|
Adjusted HR (95% CI)a)
|
No. (%)
|
Adjusted HR (95% CI)a)
|
|
Control (n=996) |
NMSC (n=249) |
Control (n=1,024) |
NMSC (n=256) |
|
Lip, oral cavity, and pharynx |
7 (0.7) |
6 (2.4) |
2.99 (1.00-9.10) |
1 (0.1) |
0 |
- |
|
Digestive organs and peritoneum |
33 (3.3) |
9 (3.6) |
0.88 (0.98-1.03) |
24 (2.3) |
3 (1.2) |
0.38 (0.11-1.26) |
|
Colorectal |
14 (1.4) |
4 (1.6) |
0.93 (0.30-2.87) |
12 (1.2) |
1 (0.4) |
0.25 (0.03-1.96) |
|
Respiratory and intrathoracic organs |
14 (1.4) |
3 (1.2) |
0.73 (0.21-2.57) |
4 (0.4) |
2 (0.8) |
1.46 (0.27-8.03) |
|
Bone, articular cartilage, connective, and other soft tissue |
0 |
3 (1.2) |
- |
2 (0.2) |
2 (0.8) |
2.85 (0.40-20.24) |
|
Melanoma |
0 |
1 (0.4) |
- |
0 |
3 (1.2) |
- |
|
Breast |
0 |
0 |
- |
6 (0.6) |
5 (2.0) |
2.58 (0.79-8.50) |
|
Genital |
10 (1.0) |
2 (0.8) |
0.76 (0.16-3.53) |
6 (0.6) |
1 (0.4) |
0.54 (0.06-4.49) |
|
Genitourinary organs |
7 (0.7) |
7 (2.8) |
3.51 (1.21-10.17) |
2 (0.2) |
2 (0.8) |
3.21 (0.45-22.98) |
|
Prostate |
9 (0.9) |
2 (0.8) |
0.86 (0.18-4.06) |
- |
- |
- |
|
Eyes |
0 |
1 (0.4) |
- |
0 |
1 (0.4) |
- |
|
Brain and nervous system |
0 |
0 |
- |
1 (0.1) |
0 |
- |
|
Thyroid gland and endocrine glands |
2 (0.2) |
0 |
- |
1 (0.1) |
0 |
- |
|
Lymphatic and hematopoietic tissue |
5 (0.5) |
1 (0.4) |
0.7 (0.08-6.12) |
2 (0.2) |
0 |
- |

Go to :

Discussion
To the best of our knowledge, this study was the first to examine the relation between NMSC and subsequent malignancy in Asians. This study found only men with NMSC had a two-fold risk of second primary cancer compared to controls, while women with NMSC did not. When further analyzed according to the involved sites, the increased risk of second primary cancer in men with NMSC was attributable to those involving the lip, oral cavity, and pharynx as well as the genitourinary organs.
Overall our study found NMSC was associated with an increased risk of only few second primary cancers, while other studies in the Western countries reported associations of NMSC with a larger number of second primary cancers. Our findings are in congruent with a Norwegian study which found men with squamous cell carcinoma had an increased risk of second primary cancer involving the mouth, pharynx, salivary glands, and prostate [
3]. However, the Norwegian study also found an additional increased risk of melanoma, hematological malignancies, and other second primary cancers involving the lung and pancreas [
3]. Similar to our findings, a Canadian study also found men with NMSC had an increased risk of second primary cancer involving the lip, oral cavity, and pharynx as well as the kidney [
13]. Nevertheless, the Canadian study did not an increase in the risk of genital organs in men with NMSC. The Canadian study also found an increased risk of second primary cancer involving more than 20 other organs [
13].
In contrast to our findings, an U.S. study found no increased risk of second primary cancer involving the lip, oral cavity, pharynx, and genitourinary organs in men with NMSC [
4]. The U.S. study identified an increased risk of melanoma in people with NMSC and an increased risk of breast cancer and lung cancer in women with NMSC [
4].
People with skin cancer have been assumed to have a higher exposure to ultraviolet radiation that promotes the synthesis of vitamin D which reduces the risk of prostate, breast, and colorectal cancer [
5]. However, our study found no significant differences in the risk of prostate, breast, and colorectal cancer between people with and without NMSC, which was similar to the findings of a U.S. study [
17]. By contrast, a Dutch study illustrated a decreased risk of developing colorectal cancer in people with skin cancer [
6], and an U.K. study reported a lower risk of breast, and prostate cancers in those with basal cell carcinoma [
18]. Meanwhile a Swiss study found the risk of prostate, breast, and colorectal cancer was escalated in people with skin cancer [
5], and a U.S. study revealed an increased risk of breast and colorectal cancer in those with skin cancer [
19]. The contradictory findings from various studies indicate the pathogenesis of prostate, breast, and colorectal cancer is complicated and cannot be explained by vitamin D alone.
In this study only men with NMSC had an increased risk of second primary cancers while women did not. To the best of our knowledge, all previous studies reported an increased risk of subsequent primary cancers in both genders. The cause for the male sex being an effect modifier in this study was unclear. Men are known to be more likely than women to own unhealthy lifestyles of smoking and alcohol drinking in Taiwan [
20], and may thus have a higher risk of having certain cancers for example cancer of the urinary tract [
21]. However, we could not adjust these lifestyle confounders in our analysis due to the lack of relevant data in the NHIRD.
The cause for various presentations of second primary cancer in people with NMSC across the world is unclear, which may be attributed to different genetic and environmental factors across various ethnic groups. For example, the incidence of prostate, breast, and colorectal cancer in Asians is much lower than that in the Western countries [
22]. The lower background incidence of these cancers might have led to the lack of significant differences in the corresponding risk between people with and without NMSC in this study.
All the three Western studies used analysis involving standardization of incidence and could not adequately adjust for potential confounders [
3,
4,
13]. Our study adjusted a range of potential confounders including the age, urbanization and CCI, and thus obtained less biased risk estimates than previous studies. Another strength of our study was the lengthy study period of up to 12 years, which allowed us to examine the long-term impact of NMSC on the risk of second primary cancers.
This study has several few limitations. Firstly, the sample size of this study was limited and some finding might be the result of random chance. Further studies are warranted to confirm our findings. Secondly, misclassification bias might exist as a few subjects with NMSC might not apply for catastrophic illness record. Also, those with second primary cancer might have not applied for catastrophic illness record as well. However, such misclassification only led to an underestimation of the observed increased risk of second primary cancer in those with NMSC. Thirdly, there were a few factors that might have affected the development of second primary cancer but we were unable to control due to lack of relevant data, for example, family history of cancer, exposure of carcinogens, co-existence of inflammatory disease (such as Crohn disease and psoriasis), metabolic syndrome, and use of immunosuppressants. However, we have mitigated the selection bias by use of propensity score matching by age, sex, and urbanization in selecting controls, use of the same index date for NMSC cases and controls, and adjustment of potential confounders including CCI in the analysis.
In conclusion, people with NMSC have an increased risk of second primary cancer. We found Asian men with NMSC had an increased risk of second primary cancer involving the lip, oral cavity, and pharynx as well as the genitourinary organs. The results of this study can be a useful reference for health education and preventive medicine interventions for people diagnosed with NMSC. A specific checkup program for people with NMSC that includes not only skin examination but also examination of other organ systems, especially the lip, oral cavity, pharynx, and the genitourinary organs, is warranted.
Go to :







 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download