1. Huguier M, Barrier A, Valinas R, Flahault A, Adloff M, Pezet D, et al. Randomized trial of 5-fluorouracil, leucovorin and cisplatin in advanced pancreatic cancer. Hepatogastroenterology. 2001; 48:875–8.
2. Mallinson CN, Rake MO, Cocking JB, Fox CA, Cwynarski MT, Diffey BL, et al. Chemotherapy in pancreatic cancer: results of a controlled, prospective, randomised, multicentre trial. Br Med J. 1980; 281:1589–91.

3. Palmer KR, Kerr M, Knowles G, Cull A, Carter DC, Leonard RC. Chemotherapy prolongs survival in inoperable pancreatic carcinoma. Br J Surg. 1994; 81:882–5.

4. Brieau B, Dahan L, De Rycke Y, Boussaha T, Vasseur P, Tougeron D, et al. Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: a large multicenter study by the Association des Gastro-Enterologues Oncologues. Cancer. 2015; 121:3290–7.
5. Fornaro L, Vivaldi C, Cereda S, Leone F, Aprile G, Lonardi S, et al. Second-line chemotherapy in advanced biliary cancer progressed to first-line platinum-gemcitabine combination: a multicenter survey and pooled analysis with published data. J Exp Clin Cancer Res. 2015; 34:156.

6. Ji JH, Song HN, Kim RB, Oh SY, Lim HY, Park JO, et al. Natural history of metastatic biliary tract cancer (BTC) patients with good performance status (PS) who were treated with only best supportive care (BSC). Jpn J Clin Oncol. 2015; 45:256–60.

7. Valle JW, Wasan H, Johnson P, Jones E, Dixon L, Swindell R, et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study: The UK ABC-01 Study. Br J Cancer. 2009; 101:621–7.
8. Park J, Kim MH, Kim KP, Park DH, Moon SH, Song TJ, et al. Natural history and prognostic factors of advanced cholangiocarcinoma without surgery, chemotherapy, or radiotherapy: a large-scale observational study. Gut Liver. 2009; 3:298–305.

9. Sharma A, Dwary AD, Mohanti BK, Deo SV, Pal S, Sreenivas V, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol. 2010; 28:4581–6.

10. Sjolander A. Propensity scores and M-structures. Stat Med. 2009; 28:1416–20.
11. Ahmann DL, Green SJ, Bisel HF, Ingle JN, Hahn RG, Lee RA, et al. An evaluation of early or delayed adjuvant chemotherapy in premenopausal patients with advances breast cancer undergoing oophorectomy: a later analysis. Am J Clin Oncol. 1982; 5:355–8.
12. Yu KD, Huang S, Zhang JX, Liu GY, Shao ZM. Association between delayed initiation of adjuvant CMF or anthracyclinebased chemotherapy and survival in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2013; 13:240.

13. Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011; 305:2335–42.
14. Des Guetz G, Nicolas P, Perret GY, Morere JF, Uzzan B. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer. 2010; 46:1049–55.

15. Gagliato Dde M, Gonzalez-Angulo AM, Lei X, Theriault RL, Giordano SH, Valero V, et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol. 2014; 32:735–44.
16. Ackland SP, Jones M, Tu D, Simes J, Yuen J, Sargeant AM, et al. A meta-analysis of two randomised trials of early chemotherapy in asymptomatic metastatic colorectal cancer. Br J Cancer. 2005; 93:1236–43.





 PDF
PDF Citation
Citation Print
Print


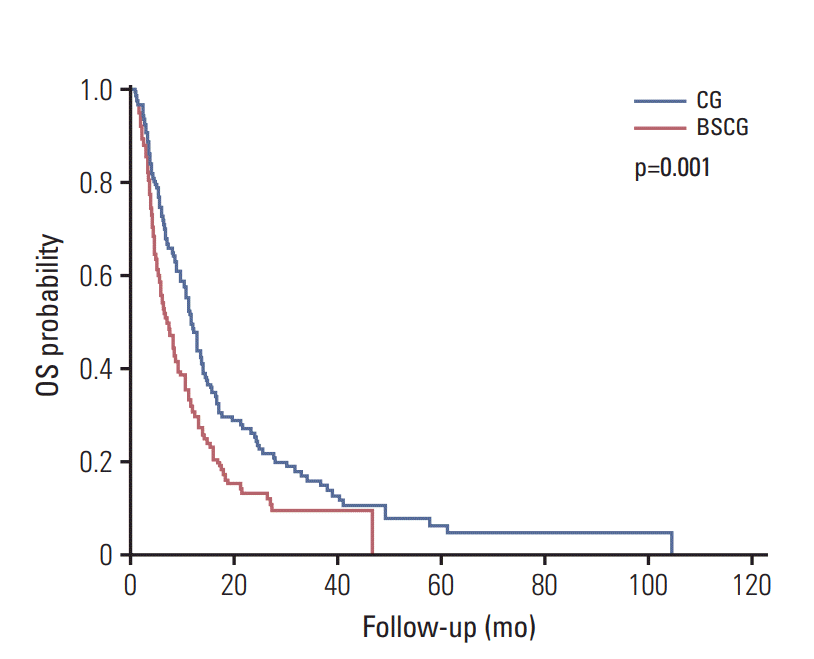
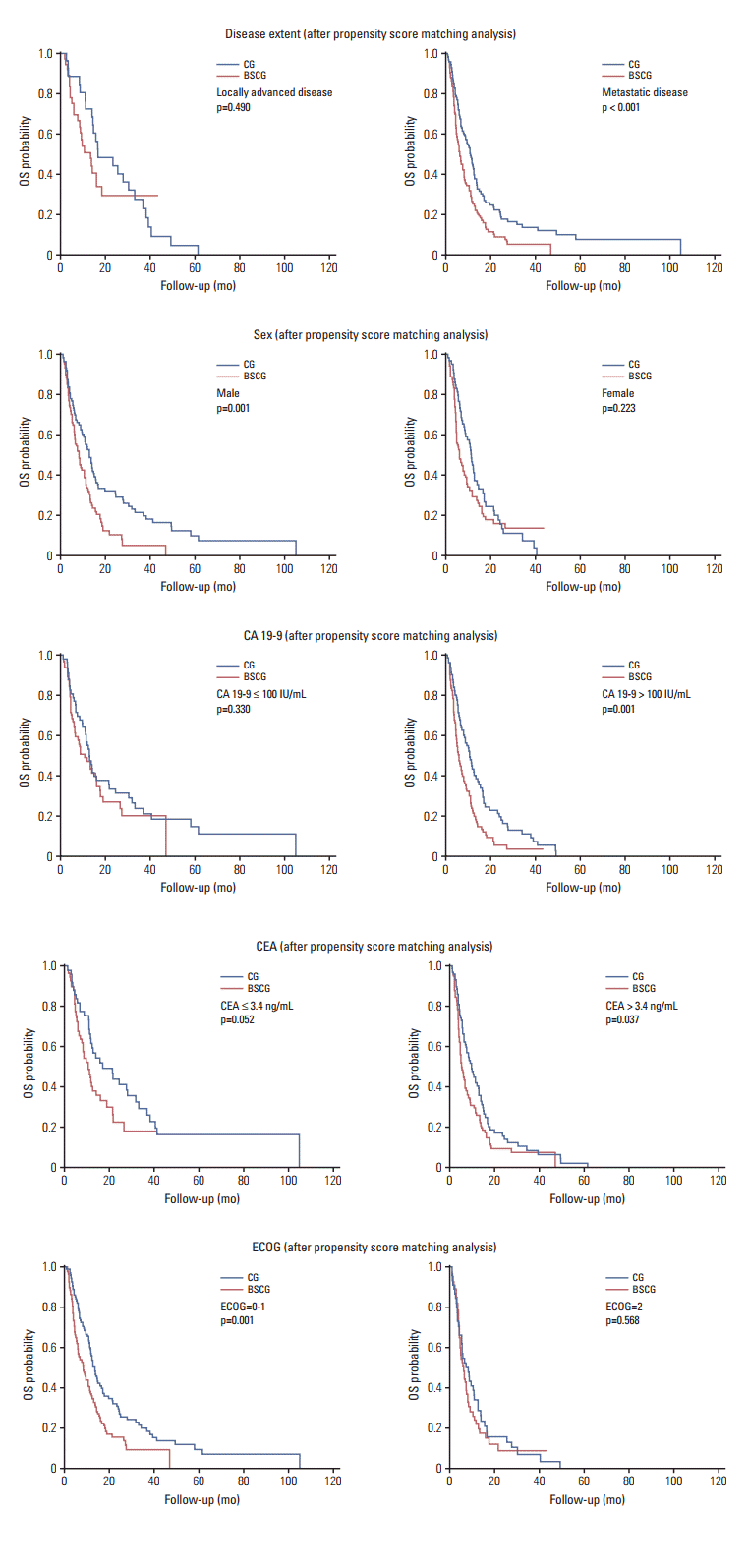
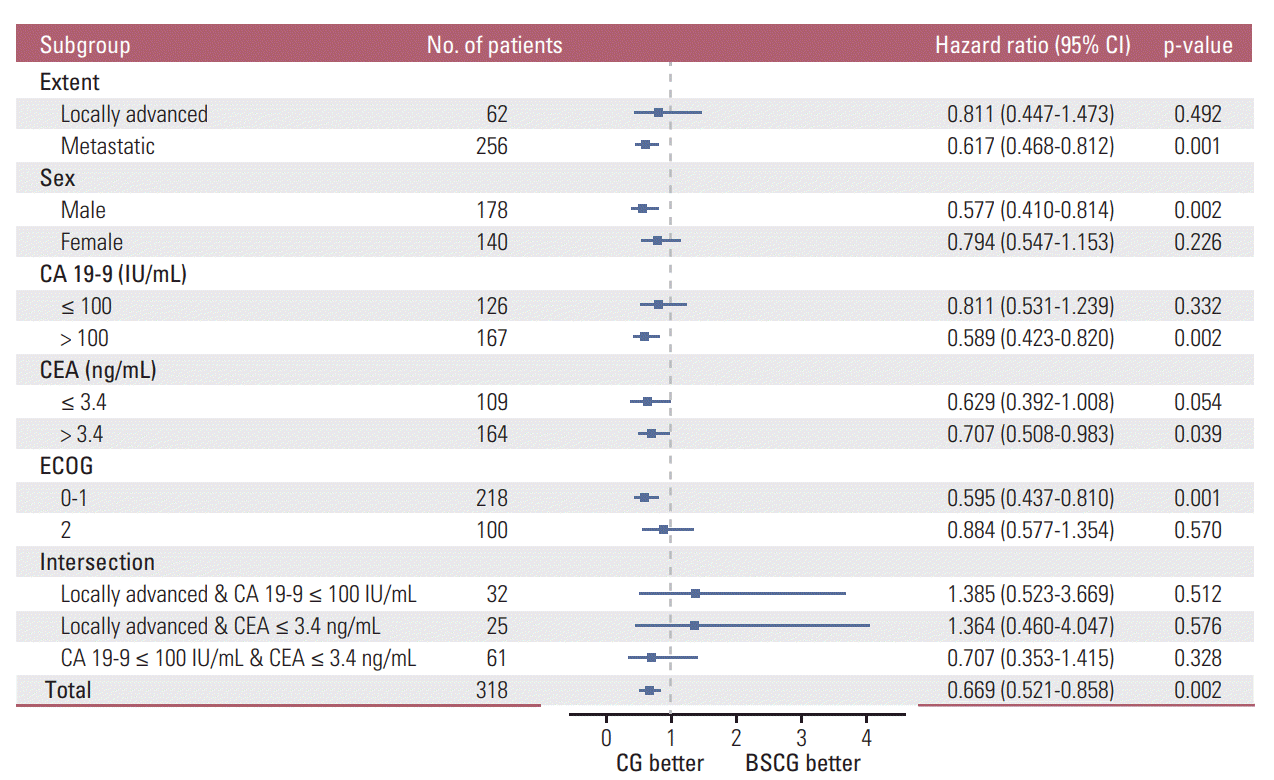
 XML Download
XML Download