Introduction
Materials and Methods
1. Cell lines
2. Preparation of the reagents
3. Determination of cell viability
4. Determination of ROS levels
5. Determination of reduced GSH levels
6. Determination of GPXs
7. Transmission electron microscopy
8. RNA Interference
9. Real-time polymerase chain reaction
10. Western blot
11. Statistical analyses
Results
1. Cisplatin induced both ferroptosis and apoptosis in A549 and HCT116 cells
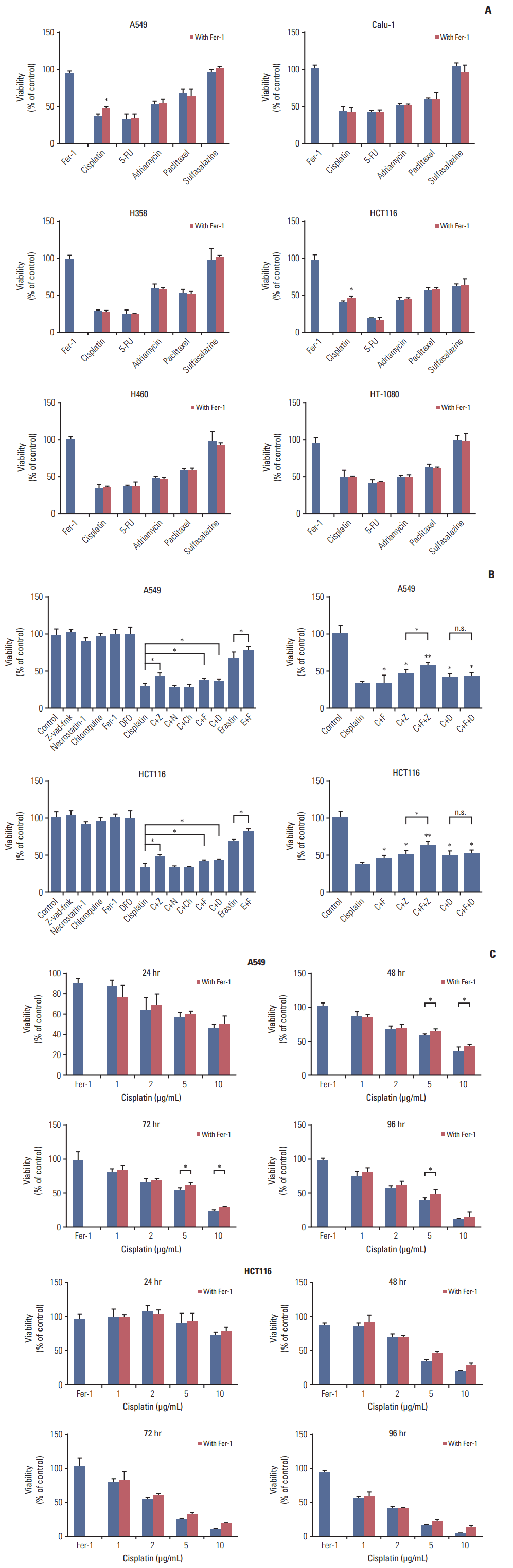 | Fig. 1.Cisplatin induced both apoptosis and ferroptosis on A549 and HCT116 cells. (A) A549, NCIH358, NCIH460, Calu-1, HCT116, and HT-1080 cells received treatments of cisplatin (5 μg/mL), fluorouracil (5-FU, 20 μg/mL), adriamycin (10 μg/mL), paclitaxel (10 μmol/L), and sulfasalazine (0.8 mmol/L), respectively in the absence or presence of ferrostatin-1 (Fer-1, 0.5 μmol/L) for 48 hours. (B) A549 and HCT116 cells were under the treatment of cisplatin (5 μg/mL) for 48 hours with different cell death inhibitors as described. C, cisplatin; Z, z-vad-fmk (20 μmol/L); N, necrostatin-1 (30 μmol/L); Ch, chloroquine (5 μg/mL); F, ferrostatin-1 (Fer-1, 0.5 μmol/L); D, deferoxamine, DFO (50 μmol/L); E, erastin (10 μmol/L); n.s., not significant. (C) A549 and HCT116 cells received treatment of cisplatin in different concentrations with or without Fer-1 (0.5 μmol/L) as displayed. Cell viabilities were analyzed by MTT. Standard error represents three independent experiments (n=3). *p < 0.05, **p < 0.01, Student’s t test. |
2. Cisplatin induced ferroptosis with proofs from multiple aspects
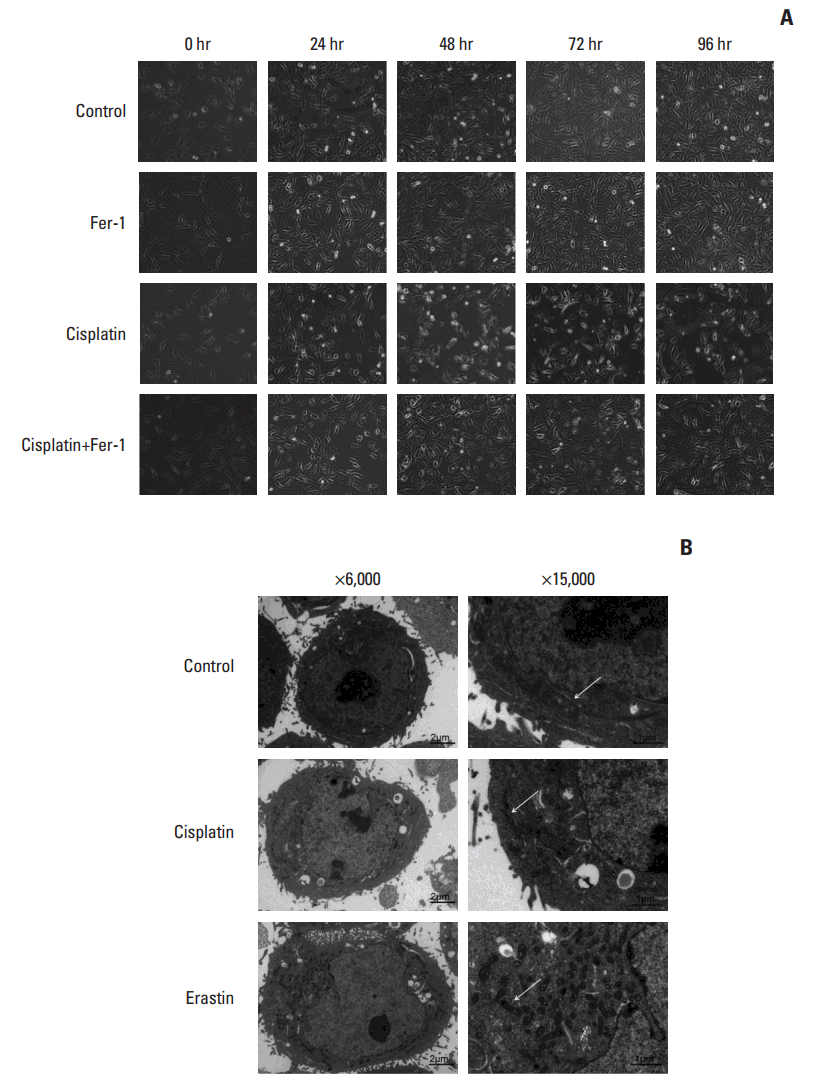 | Fig. 2.Microscopy images of cisplatin treated cells. (A) Optical microscopy images of A549 cells under the treatment of cisplatin (5 μg/mL) with or without ferrostatin-1 (Fer-1, 0.5 μmol/L) for indicated time (×100, scale bars=100 μm). (B) Transmission electron microscopy images for HCT116 cells which were treated with cisplatin (5 μg/mL) and erastin (10 μmol/L), respectively for 48 hours. Arrows indicate mitochondria in cells (left: ×6,000, scale bars=2 μm; right: ×15,000, scale bars=1 μm). |
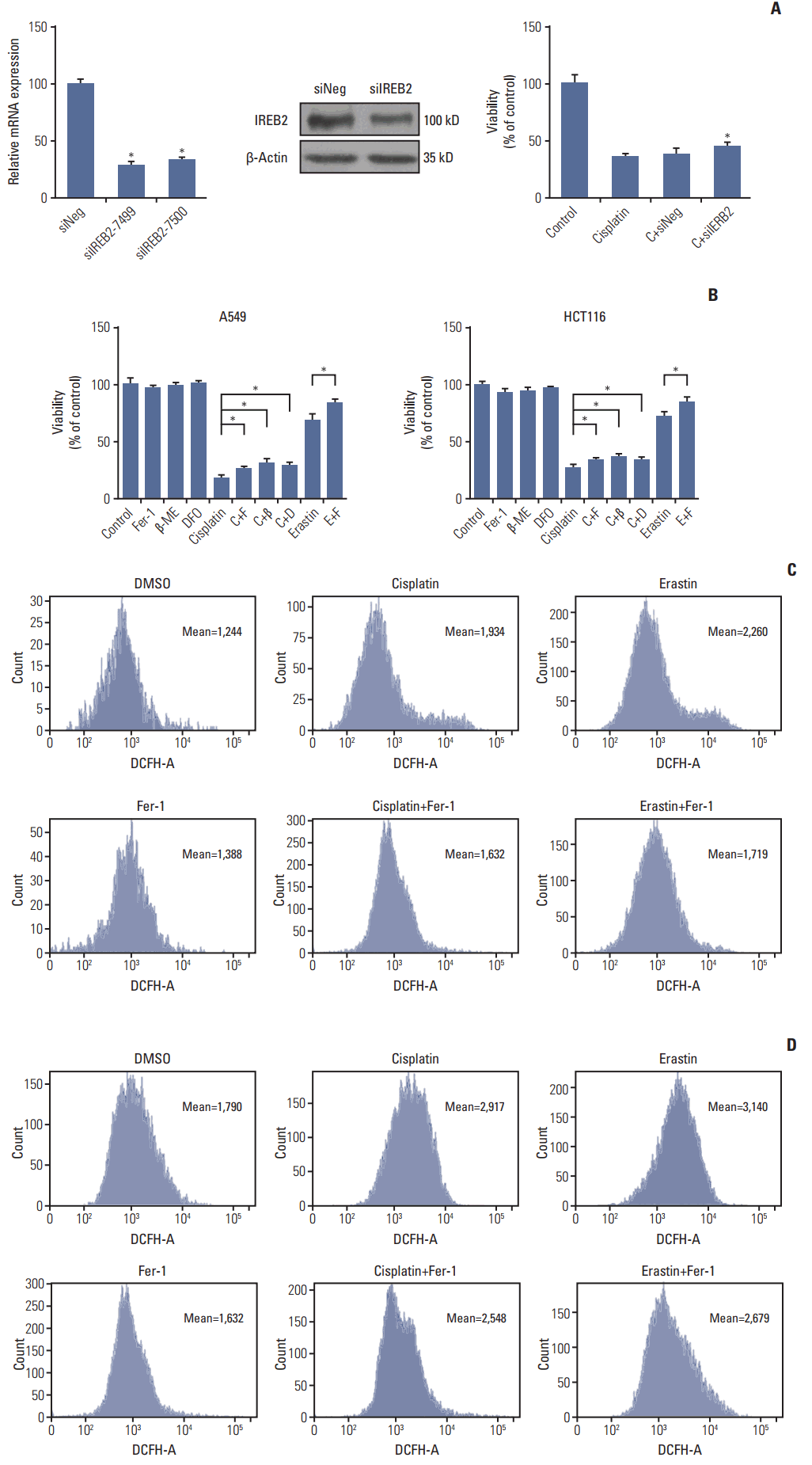 | Fig. 3.Cisplatin induced ferroptosis in A549 and HCT116 cells. (A) Polymerase chain reaction and western blot analysis of HCT116 cells transferred with small interfering RNAs (siRNAs) targeting IREB2 (siIERB2). HCT116 cells were transferred with siIERB2 or siNeg 24 hours in advance, then, treated with cisplatin (5 μg/mL) or normal saline for 48 hours. Cell viabilities were analyzed by MTT. (B) A549 and HCT116 cells were under the treatment of cisplatin (5 μg/mL) for 48 hours with different specific ferroptosis inhibitors. Cell viabilities were analyzed by MTT. Standard error represents three independent experiments (n=3). *p < 0.05, Student’s t test. C, cisplatin; F, ferrostatin-1 (Fer-1, 0.5 μmol/L); β, β-mercaptoethanol (β-ME,=50 μmol/L); D, deferoxamine (DFO, 50 μmol/L); E, erastin (10 μmol/L). (C) A549 cells were under the treatment of cisplatin (5 μg/mL), erastin (10 μmol/L), or Fer-1 (0.5 μmol/L) as demonstrated for 48 hours. Reactive oxygen species (ROS) levels in cells were evaluated and exhibited. (D) HCT116 cells were under the treatment of cisplatin (5 μg/mL), erastin (10 μmol/L), or Fer-1 (0.5 μmol/L) as demonstrated for 48 hours. ROS levels in cells were evaluated and exhibited. |
3. GSH-GPXs system involved in the underlying mechanism of cisplatin induced ferroptosis
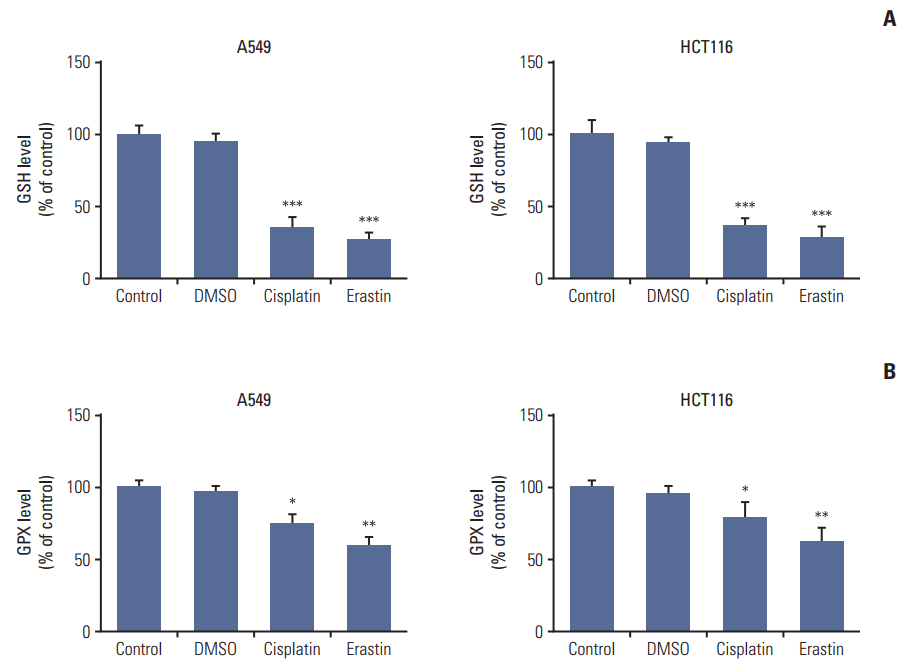 | Fig. 4.Cisplatin led to intracellular glutathione (GSH) depletion and glutathione peroxidases (GPXs) inactivation in A549 and HCT116 cells. (A) GSH level analysis of A549 and HCT116 cells. (B) GPXs activity analysis of A549 and HCT116 cells. Cells were treated with cisplatin (5 μg/mL) or erastin (10 μmol/L, as a positive control) for 48 hours, respectively. DMSO, dimethyl sulfoxide. Standard error represents three independent experiments (n=3). *p < 0.05, **p < 0.01, ***p < 0.001, Student’s t test. |
4. Additive effect observed in combination therapy of cisplatin and erastin
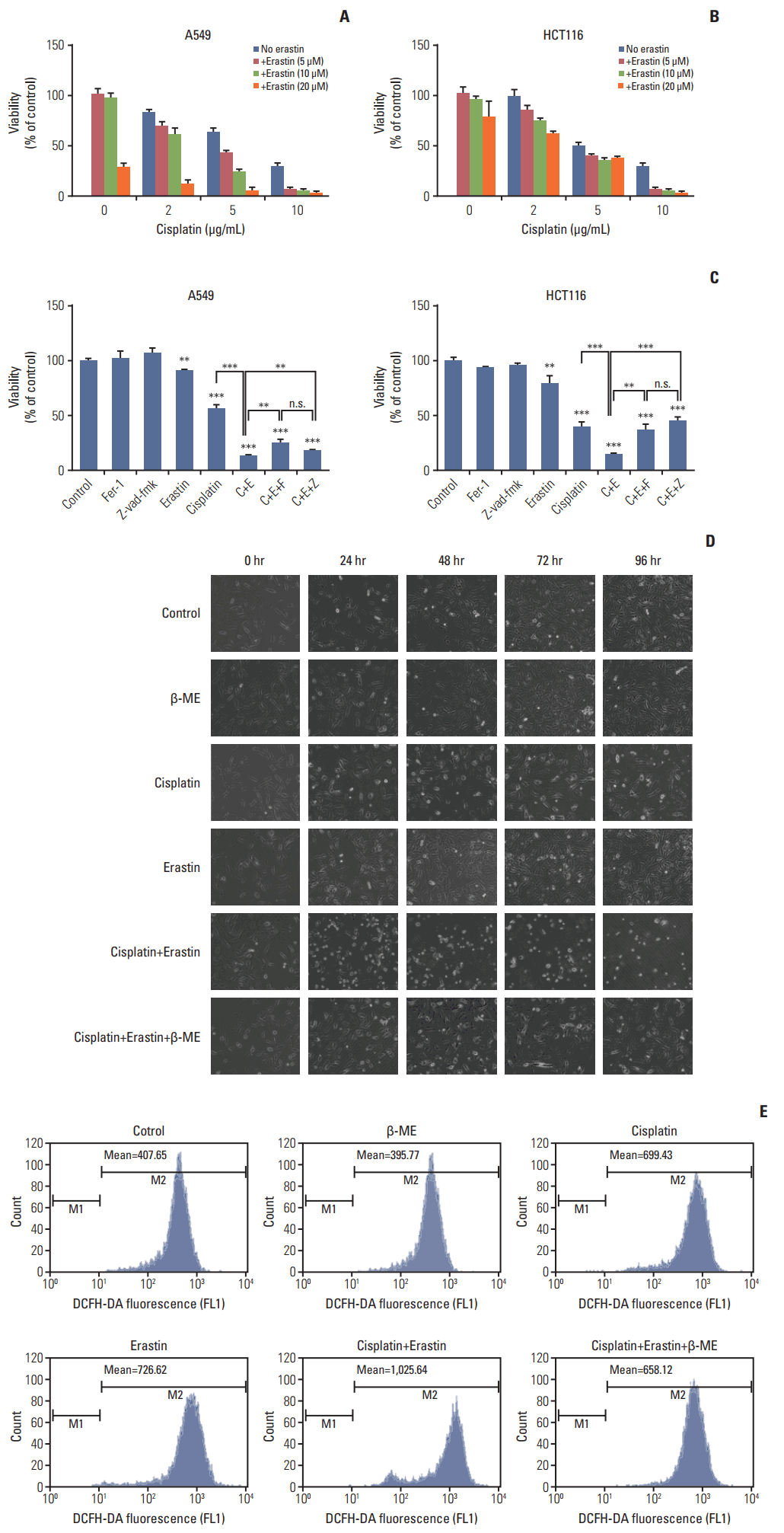 | Fig. 5.Improved anti-tumor activity was observed in combination of cisplatin and erastin. (A) A549 cells were treated with cisplatin and erastin of different concentrations as demonstrated for 48 hours. (B) HCT116 cells were treated with cisplatin and erastin of different concentrations as demonstrated for 48 hours. (C) A549 and HCT116 cells were under the treatment of cisplatin (5 μg/mL) or erastin (10 μmol/L) for 48 hours, together with z-vad-fmk (20 μmol/L) or ferrostatin-1 (Fer-1, 0.5 μmol/L), respectively. Cell viabilities were analyzed by MTT. C, cisplatin; E, erastin; F, ferrostatin-1 (Fer-1); Z, z-vad-fmk; β-ME, β-mercaptoethanol; n.s., not significant. Standard error represents three independent experiments (n=3). **p < 0.01, ***p < 0.001, Student’s t test. (D) Optical microscopy images of A549 cells under the treatment of cisplatin (5 μg/mL), erastin (10 μmol/L) with or without β-ME (50 μmol/L) for indicated time (×100, scale bars=100 μm). (E) HCT116 cells were under the treatment of cisplatin (5 μg/mL), erastin (10 μmol/L) with or without β-ME (50 μmol/L) as demonstrated for 48 hours. Reactive oxygen species levels in cells were evaluated and exhibited. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download