1. DeVita VT Jr, Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg's cancer: principles and practice of oncology. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins;2014.
2. Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005; 353:701–11.

3. Santoro A, Tursz T, Mouridsen H, Verweij J, Steward W, Somers R, et al. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 1995; 13:1537–45.
4. Elias A, Ryan L, Sulkes A, Collins J, Aisner J, Antman KH. Response to mesna, doxorubicin, ifosfamide, and dacarbazine in 108 patients with metastatic or unresectable sarcoma and no prior chemotherapy. J Clin Oncol. 1989; 7:1208–16.

5. Antman KH, Ryan L, Elias A, Sherman D, Grier HE. Response to ifosfamide and mesna: 124 previously treated patients with metastatic or unresectable sarcoma. J Clin Oncol. 1989; 7:126–31.

7. ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014; 25 Suppl 3:iii102–12.
8. Sheng JY, Movva S. Systemic therapy for advanced soft tissue sarcoma. Surg Clin North Am. 2016; 96:1141–56.

9. Ratan R, Patel SR. Chemotherapy for soft tissue sarcoma. Cancer. 2016; 122:2952–60.

10. Colosia A, Khan S, Hackshaw MD, Oglesby A, Kaye JA, Skolnik JM. A systematic literature review of adverse events associated with systemic treatments used in advanced soft tissue sarcoma. Sarcoma. 2016; 2016:3597609.

11. Bay JO, Ray-Coquard I, Fayette J, Leyvraz S, Cherix S, Piperno-Neumann S, et al. Docetaxel and gemcitabine combination in 133 advanced soft-tissue sarcomas: a retrospective analysis. Int J Cancer. 2006; 119:706–11.

12. Maki RG, Wathen JK, Patel SR, Priebat DA, Okuno SH, Samuels B, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002. J Clin Oncol. 2007; 25:2755–63.

13. Kaya AO, Buyukberber S, Ozkan M, Alkis N, Sevinc A, Ozdemir NY, et al. Efficacy and toxicity of gemcitabine plus docetaxel combination as a second line therapy for patients with advanced stage soft tissue sarcoma. Asian Pac J Cancer Prev. 2012; 13:463–7.
14. Pink D, Richter S, Gerdes S, Andreou D, Tunn PU, Busemann C, et al. Gemcitabine and docetaxel for epithelioid sarcoma: results from a retrospective, multi-institutional analysis. Oncology. 2014; 87:95–103.

15. Rapkin L, Qayed M, Brill P, Martin M, Clark D, George BA, et al. Gemcitabine and docetaxel (GEMDOX) for the treatment of relapsed and refractory pediatric sarcomas. Pediatr Blood Cancer. 2012; 59:854–8.

16. Schmitt T, Kosely F, Wuchter P, Schmier JW, Ho AD, Egerer G. Gemcitabine and docetaxel for metastatic soft tissue sarcoma: a single center experience. Onkologie. 2013; 36:415–20.
17. Lee EM, Rha SY, Lee J, Park KH, Ahn JH. Phase II study of weekly docetaxel and fixed dose rate gemcitabine in patients with previously treated advanced soft tissue and bone sarcoma. Cancer Chemother Pharmacol. 2012; 69:635–42.

18. Lee HY, Shin SJ, Kim HS, Hong SJ, Han JW, Lim ST, et al. Weekly gemcitabine and docetaxel in refractory soft tissue sarcoma: a retrospective analysis. Cancer Res Treat. 2012; 44:43–9.

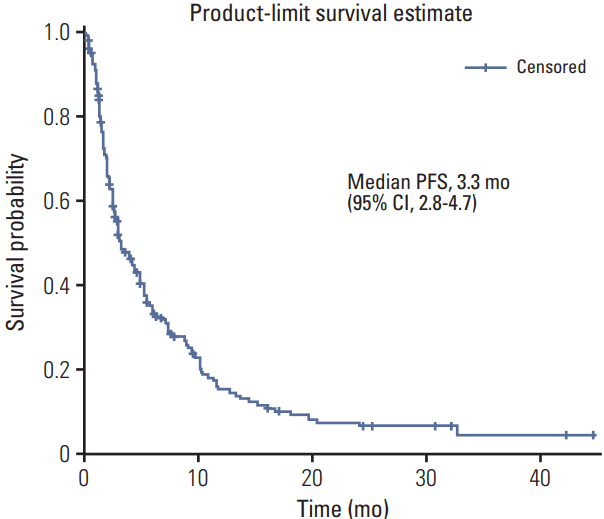




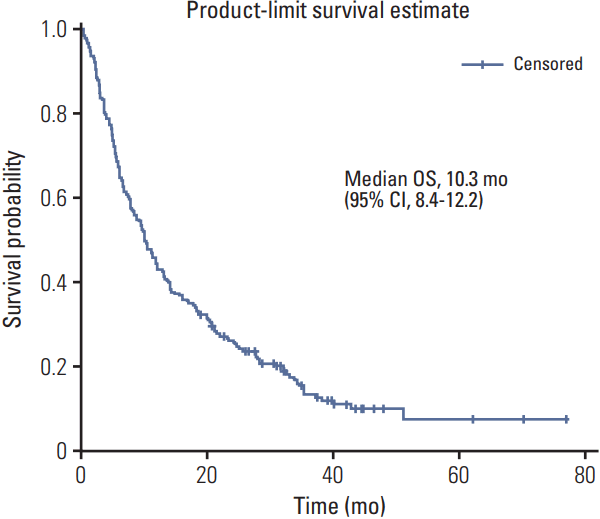
 PDF
PDF Citation
Citation Print
Print


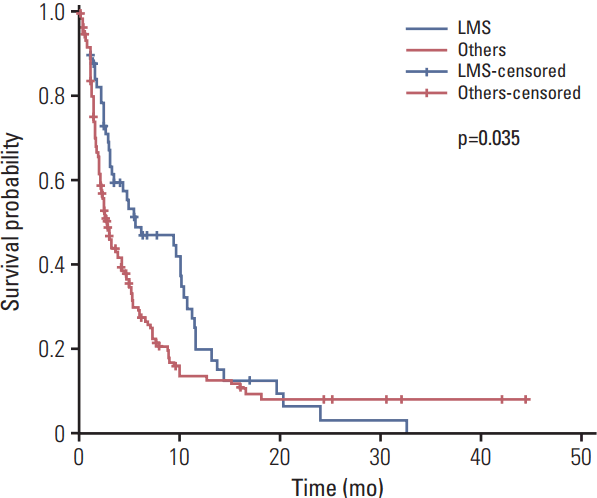
 XML Download
XML Download