Abstract
Purpose
Genexol-PM is a biodegradable cremophor EL–free polymeric micelle formulation of paclitaxel. Here,we compared efficacy and safety of Genexol-PM plus carboplatin versus Genexol plus carboplatin for ovarian cancer treatment.
Materials and Methods
In this multicenter, randomized, phase II study, patients with International Federation of Gynecology and Obstetrics IC-IV epithelial ovarian cancer were randomly assigned (1:1) to receive Genexol-PM 260 mg/m2 or Genexol 175 mg/m2 with 5 area under the curve carboplatin every 3weeks (6 cycles). The primary endpointwas the carbohydrate antigen 125 and Response Evaluation Criteria In Solid Tumor composite overall response rate (ORR).
Results
Of 131 enrolled patients, 98 were included in intention-to-treat analysis. Mean dosages were 260.00±0.00 mg/m2 Genexol-PM or 174.24±3.81 mg/m2 Genexol. Median followup was 18.0 months (range, 6.1 to 33.8 months). ORR was 88.0% (95% confidence interval [CI], 80.4 to 95.6) with Genexol-PM, and 77.1% (95% CI, 67.1 to 87.1) with Genexol (noninferiority threshold, 16.3%). Median time to progression was 14.8 months (95% CI, 11.3 to 20.2) with Genexol-PM and 15.4 months (95% CI, 13.2 to 29.6) with Genexol (p=0.550). Overall, six patients died. Neutropenia was the most common toxicity (incidences of 86.0% vs. 77.1%, p=0.120). Peripheral neuropathy incidences were 84.0% versus 64.6% (p= 0.148). Peripheral neuropathy of ≥ grade 3 occurred in one patient receiving Genexol. All toxicities were manageable.
Conclusion
Genexol-PM plus carboplatin as first-line treatment in patients with epithelial ovarian cancer demonstrated non-inferior efficacy and well-tolerated toxicities compared with the standard paclitaxel regimen. Further studies are warranted to optimize the dose and schedule, and to investigate long-term outcomes.
Ovarian cancer is the most lethal gynecologic malignancy, being responsible for approximately 14,000 deaths in the United States annually [1]. It constitutes 2.1% of cancers among Korean women [2], and over 70% of patients present with advanced stage disease. The primary treatment involves cytoreductive surgery followed by chemotherapy. Standard chemotherapy for ovarian cancer patients includes 5-7.5 area under the curve (AUC) of carboplatin combined with 175 mg/m2 of paclitaxel administered for six cycles at 3-week intervals in accordance with. Several studies demonstrate that 175 mg/m2 of paclitaxel combined with 5 AUC of carboplatin induces a Response Evaluation Criteria In Solid Tumor (RECIST) response rate of 59.5%-66% and a carbohydrate antigen 125 (CA-125) response rate of 72%-76.8% [3]. Recent reports also show that addition of bevacizumab (a monoclonal antibody against vascular endothelial growth factor) to the standard treatment prolongs progression-free survival but not overall survival (OS) [4]. Despite recent exploration of targeted therapies and immunotherapies, paclitaxel remains the most important drug in ovarian cancer treatment. Thus, many researchers are focusing on variations in the schedule and dosage with the aim of enhancing paclitaxel’s effect. The current trend is to use dose-dense weekly paclitaxel therapy; however, the effects are controversial and such treatment is associated with a higher rate of severe sensory neuropathy [5,6].
To enhance the solubility of the hydrophobic paclitaxel compound, it is prepared using polyoxyl-35-castor oil (Cremophor EL; CrEL). However, CrEL causes hypersensitivity reactions [7]. Even with premedication, paclitaxel-CrEL treatment causes minor hypersensitivity reactions in 10%-44% of patients, and potentially life-threatening reactions in 1%-3% of patients [8,9].
Genexol-PM (Samyang Co., Seoul, Korea) is a novel biodegradable polymeric micellar formulation of paclitaxel. In vivo, Genexol-PM shows greater antitumor activity and higher concentrations in tumor tissues compared to conventional paclitaxel [10]. Dose-limiting toxicities of Genexol-PM include neuropathy, myalgia, and neutropenia. Two phase I trials have tested Genexol-PM in advanced cancers; the trial performed in the United States reported a maximum tolerated dose (MTD) of 435 mg/m2, and the trial in Korea showed an MTD of 390 mg/m2. Accordingly, a Genexol-PM dose of 300 mg/m2 was recommended for phase II studies, which is higher than the recommended dose of CrEL-based paclitaxel (175 mg/m2) [11]. A phase I clinical trial was performed in Korea to investigate combination therapy with Genexol-PM plus carboplatin as a first-line treatment for ovarian cancer [12]. Although the MTD of Genexol-PM was not determined, a dosage of 260 mg/m2 was recommended for phase II trials.
Our present open-label, randomized, phase II study aimed to evaluate the efficacy and safety of first-line treatment with Genexol-PM plus carboplatin administered every 3 weeks in patients with epithelial ovarian cancer, compared to treatment with conventional CrEL-based paclitaxel (Genexol, Samyang Co.) plus carboplatin. Our results revealed that Genexol-PM was non-inferior to Genexol, and that Genexol-PM showed well-tolerated toxicities compared with the standard paclitaxel regimen. This trial was registered with ClinicalTrials.gov, number NCT01276548.
We performed a multicenter randomized, open-label, confirmatory phase II clinical trial in patients diagnosed with epithelial ovarian cancer (International Federation of Gynecology and Obstetrics [FIGO] IC-IV at diagnosis) at 10 institutions in Korea. This trial was designed to compare efficacy and safety between carboplatin combined with Genexol-PM and carboplatin combined with Genexol. The included patients had undergone debulking surgery without previous chemotherapy for treatment of epithelial ovarian cancer that was measurable on imaging according to the RECIST ver. 1.0 [13], or that showed CA-125 levels of over twice the upper limit of normal within 7 days or at two tests before starting study treatment. Additional inclusion criteria were an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2, adequate organ function, and being deemed appropriate receive paclitaxel and carboplatin for ovarian cancer treatment. Exclusion criteria were medical history of central nervous system disorder, current uncontrolled medical conditions that could interfere with the patient’s ability to undergo study treatment, preexisting sensory or motor neuropathy over grade 1 according to National Cancer Institute Common Toxicity Criteria (NCI CTCAE), and history of chemotherapy for the ovarian cancer.
All participants gave their written informed consent. The study protocol was approved by the appropriate institutional review boards and independent ethics committees, and was in compliance with Good Clinical Practice, Guidelines of the International Conference on Harmonization, and the Declaration of Helsinki.
The enrolled patients were randomly assigned (1:1) to a treatment group, with stratification according to disease status after debulking surgery in the primary setting. Either Genexol-PM and carboplatin or Genexol and carboplatin were administered on day 1 of each 3-week cycle. Drug administration began within 4 days after randomization, and was continued for a maximum of six cycles or until the investigator detected disease progression, the subject rejected the administration, or dose-limiting toxicity occurred. On day 1 of each cycle, Genexol-PM or Genexol was intravenously administered over 3 hours, followed by carboplatin administration for 30-60 minutes. Genexol-PM administration began at a dose of 260 mg/m2, Genexol was administered at a dose of 175 mg/m2, and the carboplatin dosage was 5 AUC. The Genexol-PM dosage could be increased to 300 mg/m2 starting in cycle 2 if the patient did not experience a non-hematologic adverse drug reaction of > grade 2 from the first cycle. To minimize hypersensitivity, Genexol-PM or Genexol administration was preceded by premedications, including dexamethasone and H2-blockers.
The primary efficacy outcome was the estimated composite CA-125 and RECIST response rate for each treatment group. The RECIST response rate is defined as the proportion of subjects with a measurable disease who show complete or partial response. The overall response was determined based on tumor response evaluation of target and non-target lesions after every two cycles. Tumor response and overall response were evaluated according to RECIST criteria [13]. CA-125 response rate was defined as the proportion of subjects with CA-125 response [14], which was evaluated based on responses from the initial administration to the last cycle. Each cycle included laboratory testing and assessment of ECOG performance status. Adverse events were graded following the National Cancer Institute’s CTCAE ver. 3.0.
The primary efficacy outcome was the CA-125 and RECIST composite response rate defined as the fraction of patients who showed complete responseor partial responsebased on RECIST ver. 1.0 and/or a CA-125 response. The predefined non-inferiority margin was an absolute difference of 16.3% in the primary endpoint. A one-sided 95% upper confidence limit of the difference in the objective response rate between the Genexol-PM group and the Genexol group that was below the threshold of non-inferiority was considered to indicate that the Genexol-PM group not inferior to the Genexol group. Secondary efficacy outcomes included time to progression (TTP) and OS. Categorical variables were analyzed using the chi-square test, and non-parametric variables with the Wilcoxon rank-sum test. Survival curve analysis was performed using the Kaplan-Meier method, and between-group differences were evaluated with the log-rank method. We also analyzed safety and efficacy endpoints among patients in each group who received at least one dose of study treatment. All statistical analyses were performed using SPSS ver. 21 (IBM Corp., Armonk, NY), and applying a two-sided significance level of 5%.
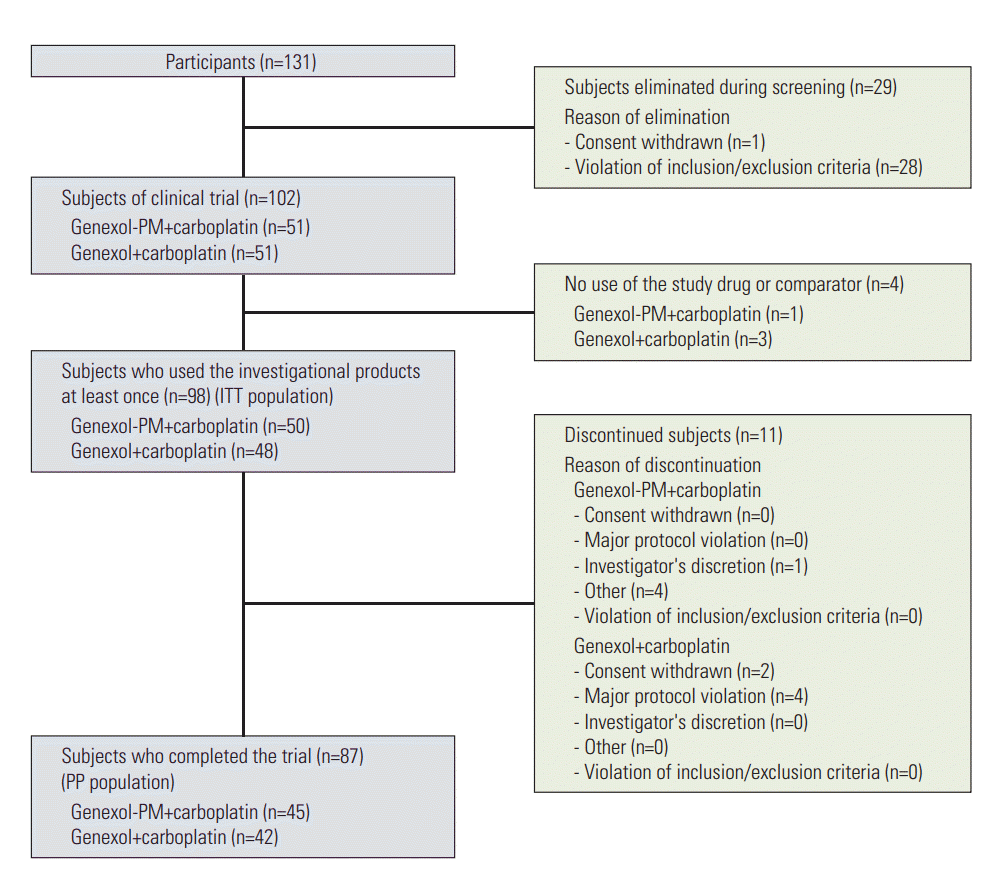
From August 2009 to May 2012, this study enrolled 131 patients with epithelial ovarian cancer, 29 patients dropped out of the screening because most of them did not meet the inclusion criteria, especially for CA-125 level more than twice the upper limit of normal after debulking surgery. Except for four patients who withdrew their consent after randomization, 98 patients were included in the analyses of clinical efficacies and safety (Fig. 1). Mean patient age was 56.4 years (±10.8 years) in the Genexol-PM group, and 55.2 years (±8.9 years) in the Genexol group (p=0.554) (Table 1). The two groups did not significantly differ with regards to histopathologic grade or FIGO stage. Over 80% of the overall subjects had a histopathological grade of G3 (poorly differentiated) or G2 (moderately differentiated). FIGO stages at diagnosis were IIIC for 38 patients (76.0%) in the study group and 31 patients (64.6%) in the control group, and IV for nine patients (18.0%) in the study group and 15 patients (31.3%) in the control group (Table 1). The mean number of cycles was 5.62±1.12 in the Genexol-PM group and 5.35±1.60 in the Genexol group. In the Genexol group, two subjects had their dose reduced once. The mean doses administered were 260.00±0.00 mg/m2 of Genexol-PM and 5.00±0.00 AUC of carboplatin in the study group, and 174.24±3.81 mg/m2 of Genexol and 5.00±0.00 AUC of carboplatin in the control group.
The study group (Genexol-PM+carboplatin) showed a CA-125 and RECIST composite overall response rate (ORR) of 88.0%, with a 95% confidence interval (CI) of 80.4-95.6. The control group (Genexol+carboplatin) showed a CA-125 and RECIST composite ORR of 77.1% (95% CI, 67.1 to 87.1). The between-groups difference in ORR was −10.9%, which was not statistically significant (p=0.701), and was lower than the non-inferiority threshold (16.3%), indicating that the Genexol-PM was not inferior to Genexol (Table 2).
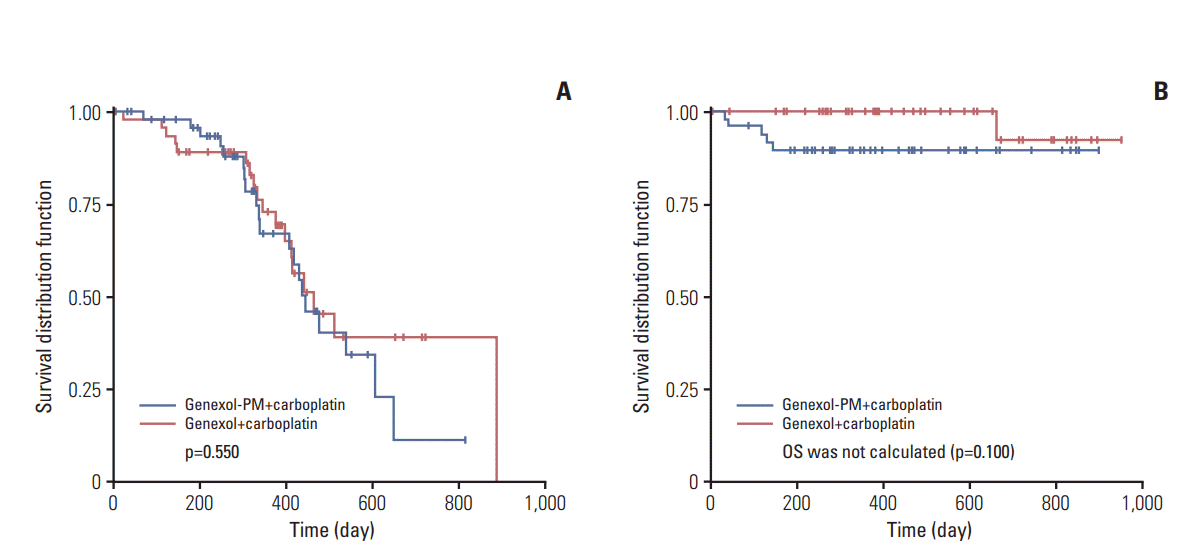
The median follow-up period was 18.0 months (range, 6.1 to 33.8 months). Tumor progression was detected in 20 patients in the Genexol-PM group, and 18 patients in the Genexol group. One patient in each group died from tumor progression. Median TTP was 14.8 months (95% CI, 11.3 to 20.2) in the Genexol-PM group and 15.4 months (95% CI, 13.2 to 29.6) in the Genexol group (p=0.550) (Fig. 2A). Deaths occurred among five subjects in the study group and one subject in the control group; however, OS with 50% mortality was not calculated. We found no significant between-groups difference in survival (p=0.100) (Fig. 2B). We analyzed the concordance between the two response rates among subjects evaluated for both CA-125 and RECIST responses. In the Genexol-PM group, 38 patients met the definition of CA-125 response, while a RECIST response was achieved by 35 subjects. In the Genexol group, 33 patients met the definition of CA-125 response, while the RECIST response was met by 29 patients.
During the study period, 441 adverse events occurred in 50 subjects (100.0%) in the Genexol-PM group, including 49 serious events (11.1%, 49/441). In the Genexol group, 376 adverse events occurred in 44 subjects (91.7%), including 40 serious events (10.6%, 40/376). Except for alopecia, the rates of hematologic and nonhematologic adverse events did not significantly differ between the two groups. Neutropenia occurred in 43 patients (86.0%) in the Genexol-PM group and 37 patients (77.1%) in the Genexol group (p=0.120), even though a higher paclitaxel dose was administered in the Genexol-PM group. In the Genexol-PM group, there were 104 confirmed instances of hematologic toxicities (23.6%, 104/441) compared to 77 instances in the Genexol group (20.5%, 77/376). Hypersensitivity reactions occurred in seven patients (14.0%) in the Genexol-PM group, and three patients (6.3%) in the Genexol group (p > 0.99). Incidences of peripheral neuropathy and myalgia did not significantly differ according to study treatment. Peripheral neuropathy occurred in 42 patients (84.0%) in the Genexol-PM group with no serious cases, and in 31 patients (64.6%) in the Genexol group with one case (2.1%) considered serious (p=0.148) (Table 3). All reported toxicities were successfully managed with conservative care, and no treatment-related deaths occurred in either group. In the Genexol-PM group, ECOG performance from screening/baseline to last observation carried forward changed by 0.02±0.68, which was not statistically significant (p=0.837).
Paclitaxel is an important drug in ovarian cancer treatment. However, the commonly used paclitaxel solubilizer CrEL contributes to severe toxicities, including hypersensitivity reactions and peripheral neuropathies [7,15-18]. To overcome these toxicities and to potentially improve efficacy, solvent-free paclitaxel formulations have been developed. Genexol-PM is a freeze-dried macromolecule micelle of paclitaxel that was approved in Korea in 2006 with the indications of first-line therapy for recurrent and metastatic breast cancer (MBC) and non-small cell lung carcinoma (NSCLC). The copolymer mPEG-PDLLA that is used in Genexol-PM is not listed in the U.S. Pharmacopoeia; however, the individual polymers mPEG and polylactide are used in multiple pharmaceutical products and are on the Food and Drug Administration's Inactive Ingredient List. The mPEG-PDLLA copolymer has been confirmed to be nontoxic. Several phase II clinical trials demonstrate that Genexol-PM is generally well tolerated and shows sufficient antitumor activity in patients with MBC, NSCLC, and urothelial carcinoma [19-21]. Phase II clinical trials have been performed in the United States to examine Genexol-PM monotherapy for the treatment of progressive pancreatic cancer [22]. In Korea, a phase I clinical trial investigated the combination of Genexol-PM with carboplatin in ovarian cancer patients (featured poster presentation, abstract 0071) [12].
Our present study evaluated the anti-tumor effects of firstline treatment with Genexol-PM+carboplatin or with Genexol+carboplatin among epithelial ovarian cancer patients, and demonstrated that the efficacy outcome in the study group was not inferior to that in the control group. Genexol-PM plus carboplatin was associated with a CA-125 and RECIST composite response rate of 88.0% in intentionto-treat (ITT) analysis (the primary efficacy outcome), and 93.2% in per-protocol analysis. The primary efficacy outcome differed between the groups by −10.92%, with a one-sided 95% upper confidence limit of 1.60%, which was below the non-inferiority threshold (16.3%)—indicating that outcome with Genexol-PM was not inferior to outcome with Genexol. As a secondary efficacy outcome, the RECIST response rate from ITT analysis of subjects with measurable lesions was 85.4% in the Genexol-PM group and 75.0% in the Genexol group. Among the subjects without measurable lesions, the CA-125 response rate was 100% in both groups. With regard to OS rate in the ITT population, five subjects died and 47 subjects were censored in the Genexol-PM group, and one subject died and 46 subjects were censored in Genexol group. OS with 50% mortality was not calculated. The betweengroup difference in survival was not statistically significant (p=0.100).
Higher doses of conventional paclitaxel do not improve response rates or survival among patients with MBC, predominantly due to greater toxicities [23]. On the other hand, compared to conventional paclitaxel, nanoparticle albuminbound (nab) paclitaxel produces higher response rates with similar safety profiles in cases of MBC, NSCLC, and pancreatic cancer [24-26]. However, controversial results are reported by several randomized trials investigating the roles of higher paclitaxel doses in breast cancer treatment [27,28]. No comparative clinical trials have evaluated the efficacy of high-dose paclitaxel in epithelial ovarian cancer. Our present phase II trial in ovarian cancer demonstrated the non-inferiority of Genexol-PM but did not show that higher paclitaxel doses improved efficacy with regards to ORR, TTP, or OS. Thus, there remains a need for additional clinical trials, particularly phase III/IV studies in a large number of patients with ovarian cancer to test the superiority of high-dose Genexol-PM compared to the conventional dose of paclitaxel. This may be a great help in selecting chemotherapeutic agents as first-line treatment for patients with ovarian cancer.
In terms of safety, as expected, the Genexol-PM group showed higher incidence of neutropenia and lower incidence of peripheral neuropathy, but in this study, Genexol-PM and conventional paclitaxel showed similar safety profiles, including neutropenia and peripheral neuropathy. Some researchers have reported that peripheral neuropathy due to paclitaxel itself is inevitable, although the solvent-free paclitaxel formulation has the advantages of inducing less severe peripheral neuropathy and more rapid recovery compared with conventional paclitaxel [24,29]. A randomized trial of nab-paclitaxel plus gemcitabine versus gemcitabine alone for treatment of pancreatic cancer found the most notable difference in adverse events to be the rate of peripheral neuropathy (17% with nab-paclitaxel plus gemcitabine versus 1% with gemcitabine) [26]. Notably, peripheral neuropathy in that study was rapidly reversible in most patients, with neuropathy of ≥ grade 3 improved to ≤ grade 1 in a median of 29 days. In our present study, no patients in the Genexol-PM group showed peripheral neuropathy of grade 3 or higher.
In conclusion, our present phase II study demonstrated a non-inferior ORR and manageable toxicities with Genexol-PM plus carboplatin compared to with standard Genexol and carboplatin. Genexol-PM allows administration of a greater paclitaxel dose without compromising patient safety. Overall, Genexol-PM with carboplatin seems to be efficacious and safe as first-line therapy in patients with epithelial ovarian cancer. Further studies of Genexol-PM are warranted, particularly to examine the use of different schedules, including weekly doses, and to study long-term outcomes, including the reversibility of peripheral neuropathy.
ACKNOWLEDGMENTS
We thank all of the patients and their families who participated in this phase II study, the study-site staff who assisted with coordination and medical monitoring, and Dr. Paul Kretchmer (English Editor, San Francisco Edit) for medical writing assistance.
References
2. Korea Central Cancer Registry, National Cancer Center. Annual report of cancer statistics in Korea in 2012. Goyang: National Cancer Center;2014.
3. Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003; 21:3194–200.

4. Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011; 365:2473–83.

5. Katsumata N, Yasuda M, Isonishi S, Takahashi F, Michimae H, Kimura E, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol. 2013; 14:1020–6.

6. Chan JK, Brady MF, Penson RT, Huang H, Birrer MJ, Walker JL, et al. Weekly vs. every-3-week paclitaxel and carboplatin for ovarian cancer. N Engl J Med. 2016; 374:738–48.

7. Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001; 37:1590–8.
8. Kintzel PE. Prophylaxis for paclitaxel hypersensitivity reactions. Ann Pharmacother. 2001; 35:1114–7.

9. Kloover JS, den Bakker MA, Gelderblom H, van Meerbeeck JP. Fatal outcome of a hypersensitivity reaction to paclitaxel: a critical review of premedication regimens. Br J Cancer. 2004; 90:304–5.

10. Kim SC, Kim DW, Shim YH, Bang JS, Oh HS, Kim SW, et al. In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy. J Control Release. 2001; 72:191–202.

11. Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, Heo DS, et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res. 2004; 10:3708–16.

12. Abstracts of the 43rd Annual Meeting of the Society of Gynecologic Oncology. March 24-27, 2012. Austin, Texas, USA. Gynecol Oncol. 2012; 125 Suppl 1:S2–188.
13. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–16.
14. Rustin GJ. Use of CA-125 to assess response to new agents in ovarian cancer trials. J Clin Oncol. 2003; 21(10 Suppl):187s–93s.

17. Sparreboom A, van Zuylen L, Brouwer E, Loos WJ, de Bruijn P, Gelderblom H, et al. Cremophor EL-mediated alteration of paclitaxel distribution in human blood: clinical pharmacokinetic implications. Cancer Res. 1999; 59:1454–7.
18. van Zuylen L, Karlsson MO, Verweij J, Brouwer E, de Bruijn P, Nooter K, et al. Pharmacokinetic modeling of paclitaxel encapsulation in Cremophor EL micelles. Cancer Chemother Pharmacol. 2001; 47:309–18.

19. Lee KS, Chung HC, Im SA, Park YH, Kim CS, Kim SB, et al. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res Treat. 2008; 108:241–50.

20. Kim DW, Kim SY, Kim HK, Kim SW, Shin SW, Kim JS, et al. Multicenter phase II trial of Genexol-PM, a novel Cremophorfree, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Ann Oncol. 2007; 18:2009–14.

21. Lee JL, Ahn JH, Park SH, Lim HY, Kwon JH, Ahn S, et al. Phase II study of a cremophor-free, polymeric micelle formulation of paclitaxel for patients with advanced urothelial cancer previously treated with gemcitabine and platinum. Invest New Drugs. 2012; 30:1984–90.

22. Saif MW, Podoltsev NA, Rubin MS, Figueroa JA, Lee MY, Kwon J, et al. Phase II clinical trial of paclitaxel loaded polymeric micelle in patients with advanced pancreatic cancer. Cancer Invest. 2010; 28:186–94.

23. Winer EP, Berry DA, Woolf S, Duggan D, Kornblith A, Harris LN, et al. Failure of higher-dose paclitaxel to improve outcome in patients with metastatic breast cancer: cancer and leukemia group B trial 9342. J Clin Oncol. 2004; 22:2061–8.

24. Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005; 23:7794–803.

25. Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okamoto I, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012; 30:2055–62.

26. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013; 369:1691–703.

27. Rugo HS, Barry WT, Moreno-Aspitia A, Lyss AP, Cirrincione C, Leung E, et al. Randomized phase III trial of paclitaxel once per week compared with nanoparticle albumin-bound Nabpaclitaxel once per week or ixabepilone with bevacizumab as first-line chemotherapy for locally recurrent or metastatic breast cancer: CALGB 40502/NCCTG N063H (Alliance). J Clin Oncol. 2015; 33:2361–9.

Table 1.
Patient characteristics
Table 2.
Responses and survival rates
| Response rate |
Genexol-PM+carboplatin (n=50) |
Genexol+carboplatin (n=48) |
p-value | ||
|---|---|---|---|---|---|
| No. (%) or median | 95% CI | No. (%) or median | 95% CI | ||
| Overall responsea) | 44 (88.0) | 80.4-95.6 | 37 (77.1) | 67.1-87.1 | 0.701 |
| CR | 24 (48.0) | 16 (33.3) | |||
| PR | 20 (40.0) | 21 (43.8) | |||
| SD | 3 (6.0) | 5 (10.4) | |||
| PD | 0 | 1 (2.1) | |||
| NE | 3 (6.0) | 4 (8.3) | |||
| Survival rate | |||||
| Time to progressionb) | 14.8 | 11.3-20.2 | 15.4 | 13.2-29.6 | 0.546 |
| Overall survivalc) | (–) | (–) | 0.100 | ||
Table 3.
Overview of adverse events




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download