Abstract
Purpose
Materials and Methods
Results
Conclusion
Electronic Supplementary Material
Notes
*Korean Breast Cancer Society
Sei Hyun Ahn1, Dong-Young Noh2, Seok Jin Nam3, Sung Yong Kim3, Eun Sook Lee4, Byeong-Woo Park5, Woo Chul Noh6, Jung Han Yoon7, Soo Jung Lee8, Eun Kyu Lee9, Joon Jeong10, Sehwan Han11, Ho Yong Park12, Nam-Sun Paik13, Young Tae Bae14, Hyouk Jin Lee15, Heung kyu Park16, Seung Sang Ko17, Byung Joo Song18, Young Jin Suh19, Sung Hoo Jung20, Se Heon Cho21, Sei Joong Kim22, Se Jeong Oh23, Byung Kyun Ko24, Ku Sang Kim25, Chanheun Park26, Jong-Min Baek27, Ki-Tae Hwang28, Il-Sung Chang29, Jeoung Won Bae30, Jeong-Soo Kim31, Sun Hee Kang32, Geumhee Gwak33, Jee Hyun Lee34, Tae Hyun Kim35, Myungchul Chang36, Jung Sun Lee37, Jeong-Yoon Song38, Hai Lin Park39, Sun Young Min40, Jung-Hyun Yang41, Sung Hwan Park42, Woo-Chan Park43, Lee Su Kim44, Dong Won Ryu45, Kweon Cheon Kim46, Min Sung Chung47, Hee Boong Park48, Cheol Wan Lim49, Un Jong Choi50, Beom Seok Kwak51, Young Sam Park52, Hyuk Jai Shin53, Young Jin Choi54, Doyil Kim55, Airi Han56, Jong Hyun Koh57, Sangyong Choi58, Daesung Yoon59, Soo Youn Choi60, Shin Hee Chul61, Jae Il Kim62, Jae Hyuck Choi63, Jin Woo Ryu64, Chang Dae Ko65, Il Kyun Lee66, Dong Seok Lee67, Seunghye Choi68, Youn Ki Min69, Young San Jeon70, Eun-Hwa Park71
1Asan Medical Center, 2Seoul National University Hospital, 3Soonchunhyang University Cheonan Hospital, 4National Cancer Center; 5Yonsei University Severance Hospital, 6Korea Cancer Center Hospital, 7 Chonnam National University Hwasun Hospital, 8 Yeungnam University Medical Center, 9Seoul National University Bundang Hospital, 10 Yonsei University Gangnam Severance Hospital, 11Ajou University School of Medicine, 12Kyungpook National University Medical Center, 13Ewha Womans University Mokdong Hospital, 14Pusan National University Hospital, 15Saegyaero Hospital, 16 Gachon University Gil Hospital, 17 Cheil General Hospital and Women’s Healthcare Center, Dankook University College of Medicine, 18 Seoul St. Mary’s Hospital, The Catholic University of Korea, 19 St. Vincent’s Hospital, The Catholic University of Korea, 20 Chonbuk National University Hospital, 21 Dong-A University Medical Center, 22 Inha University Hospital, 23 Incheon St. Mary’s Hospital, The Catholic University of Korea, 24 Ulsan University Hospital, 25 Ulsan City Hospital, 26 Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, 27 Bucheon St. Mary’s Hospital, The Catholic University of Korea, 28SMG-SNU Boramae Medical Center, 29Chungnam National University Hospital, 30Korea University Anam Hospital, 31Uijeongbu St. Mary’s Hospital, The Catholic University of Korea, 32Keimyung University Dongsan Medical Center, 33Inje University Sanggye Paik Hospital, 34Soonchunhyang University Hospital, 35Inje University Busan Paik Hospital, 36Dankook University Hospital, 37Inje University Haeundae Paik Hospital, 38Kyung Hee University Hospital at Gangdong, 39CHA Gangnam Medical Center, CHA University, 40Kyung Hee University Medical Center, 41Konkuk University Medical Center, 42Daegu Catholic University Medical Center, 43Yeouido St. Mary’s Hospital, The Catholic University of Korea, 44Hallym University Sacred Heart Hospital, 45Kosin University Gospel Hospital, 46Chosun University Hospital, 47Hanyang University Seoul Hospital, 48Park Hee Boong Surgical Clinic, 49Soonchunhyang University Bucheon Hospital, 50Wonkwang University Hospital, 51Dongguk University Ilsan Hospital, 52Presbyterian Medical Center, 53Myongji Hospital, 54Chungbuk National University Hospital, 55MizMedi Hospital, 56Wonju Severance Christian Hospital, Yonsei University College of Medicine, 57Cheongju St. Mary’s Hospital, 58Gwangmyung Sung Ae Hospital, 59Konyang University Hospital, 60Hallym University Kangdong Sacred Heart Hospital, 61Chung-Ang University Hospital, 62Inje University Ilsan Paik Hospital, 63 Jeju National University Hospital, 64Chungmu Genral Hospital, 65Dr. Ko’s Breast Clinic, 66International St. Mary’s Hospital, Catholic Kwandong University College of Medicine, 67Bun Hong Hospital, 68St. Paul's Hospital, The Catholic University of Korea, 69Cheju Halla General Hospital, 70Goo Hospital, 71Gangneung Asan Hospital, University of Ulsan College of Medicine
References
Fig. 1.
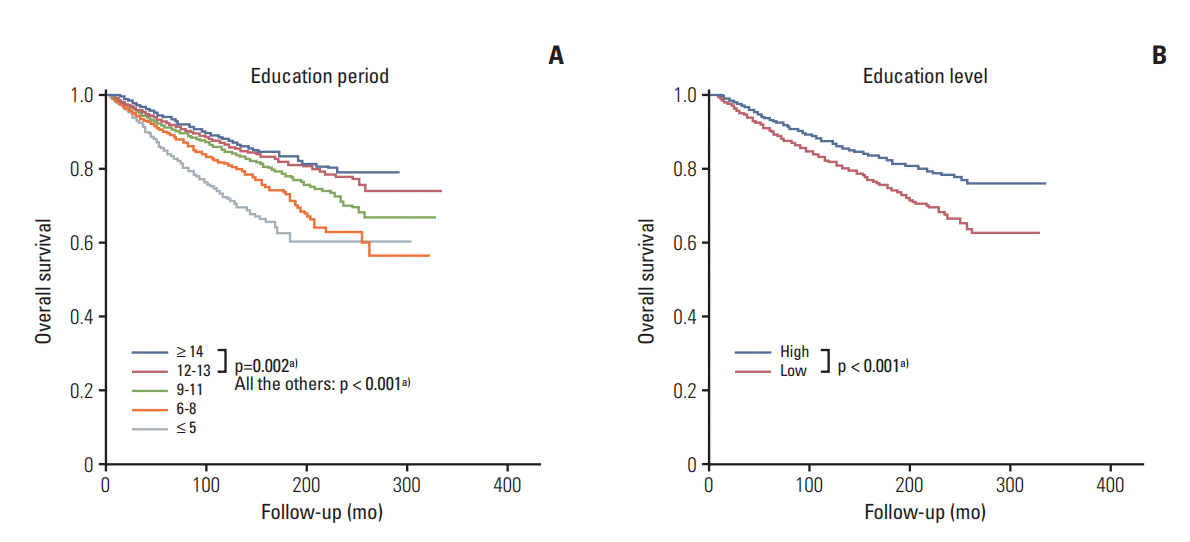
Fig. 2.
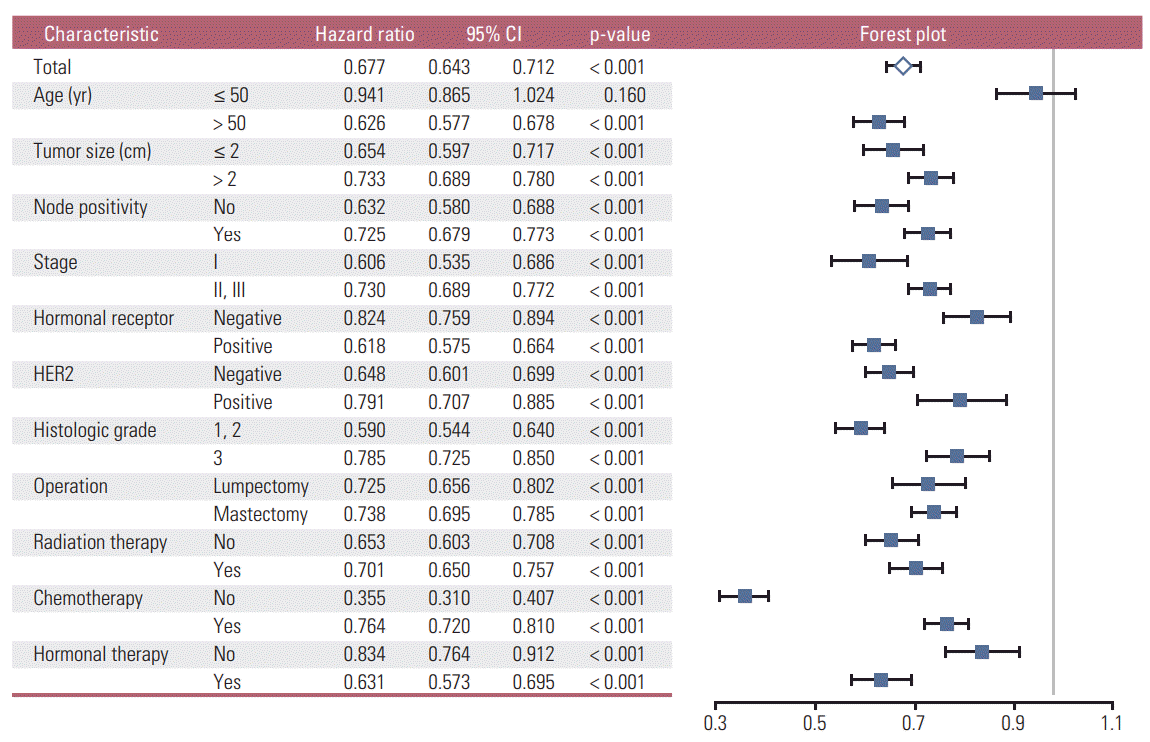
Fig. 3.
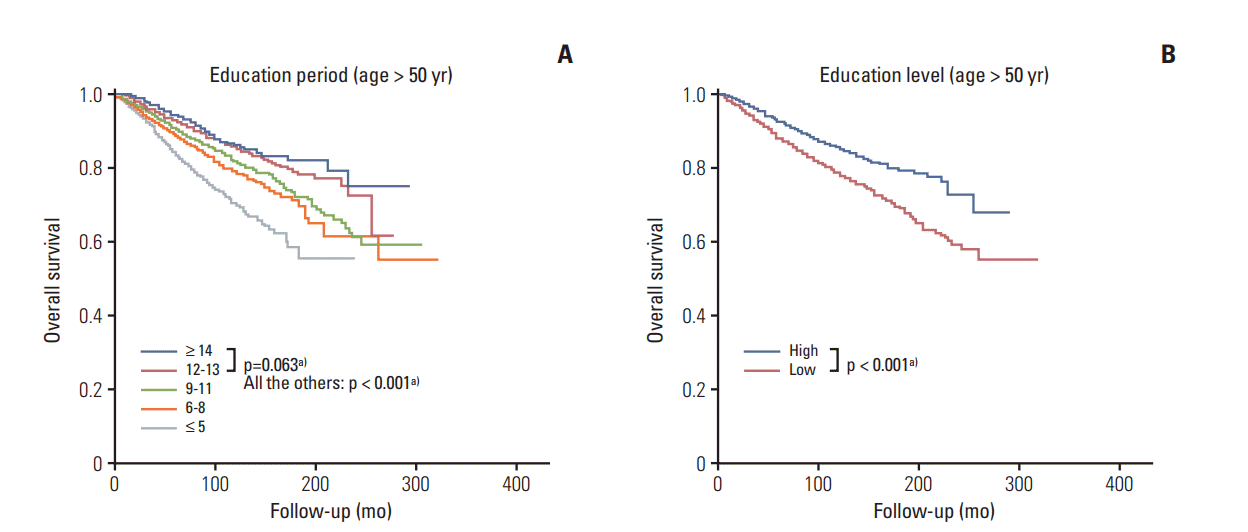
Table 1.
| Characteristic |
Education period |
Education level |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤ 5 | 6-8 | 9-11 | 12-13 | ≥ 14 | Total | p for trend | Low | High | Chi-squarea) p-value | |
| Total | 2,035 (3.2) | 8,434 (13.2) | 9,955 (15.5) | 25,464 (39.7) | 18,241(28.4) | 64,129 (100) | 20,424 (31.8) | 43,705 (68.2) | ||
| Mean age (yr) | 64.39±10.355 | 59.58±9.591 | 53.3±8.825 | 47.67±8.956 | 44.54±9.062 | 49.75±10.662 | < 0.001 | 57.00±10.075 | 46.37±9.132 | < 0.001 |
| Age (yr) | ||||||||||
| ≤ 50 | 227 (11.2) | 1,544 (18.3) | 4,161 (41.8) | 17,244 (67.7) | 14,358 (78.7) | 37,534 (58.5) | < 0.001 | 5,932 (29.0) | 31,602 (72.3) | < 0.001 |
| > 50 | 1,808 (88.8) | 6,888 (81.7) | 5,793 (58.2) | 8,219 (32.3) | 3,882 (21.3) | 26,590 (41.5) | 14,489 (71.0) | 12,101 (27.7) | ||
| Tumor size (cm) | ||||||||||
| ≤ 2 | 981 (49.1) | 4,385 (53.0) | 5,306 (54.3) | 14,268 (57.2) | 10,688 (60.4) | 35,628 (56.9) | < 0.001 | 10,672 (53.2) | 24,956 (58.6) | < 0.001 |
| > 2 | 1,016 (50.9) | 3,886 (47.0) | 4,472 (45.7) | 10,659 (42.8) | 6,998 (39.6) | 27,031 (43.1) | 9,374 (46.8) | 17,657 (41.4) | ||
| Node positivity | ||||||||||
| No | 1,185 (59.2) | 5,135 (61.6) | 6,140 (62.2) | 15,976 (63.4) | 11,748 (65.3) | 40,184 (63.4) | < 0.001 | 12,460 (61.6) | 27,724 (64.2) | < 0.001 |
| Yes | 815 (40.8) | 3,201 (38.4) | 3,737 (37.8) | 9,235 (36.6) | 6,248 (34.7) | 23,236 (36.6) | 7,753 (38.4) | 15,483 (35.8) | ||
| Stage | ||||||||||
| I | 708 (35.9) | 3,282 (40.0) | 4,021 (41.3) | 10,888 (43.9) | 8,286 (47.1) | 27,185 (43.7) | < 0.001 | 8,011 (40.2) | 19,174 (45.3) | < 0.001 |
| II, III | 1,263 (64.1) | 4,926 (60.0) | 5,709 (58.7) | 13,893 (56.1) | 9,292 (52.9) | 35,083 (56.3) | 11,898 (59.8) | 23,185 (54.7) | ||
| Hormonal receptor | ||||||||||
| Negative | 590 (32.3) | 2,573 (33.9) | 2,971 (32.8) | 6,589 (28.1) | 4,442 (26.2) | 17,165 (29.2) | < 0.001 | 6,134 (33.2) | 11,031 (27.3) | < 0.001 |
| Positive | 1,238 (67.7) | 5,010 (66.1) | 6,084 (67.2) | 16,818 (71.9) | 12,521 (73.8) | 41,671 (70.8) | 12,332 (66.8) | 29,339 (72.7) | ||
| HER2 | ||||||||||
| Negative | 1,105 (74.9) | 4,632 (73.0) | 5,033 (70.9) | 14,628 (75.3) | 11,159 (77.6) | 36,557 (75.0) | < 0.001 | 10,770 (72.2) | 25,787 (76.2) | < 0.001 |
| Positive | 371 (25.1) | 1,716 (27.0) | 2,067 (29.1) | 4,808 (24.7) | 3,225 (22.4) | 12,187 (25.0) | 4,154 (27.8) | 8,033 (23.8) | ||
| Histologic grade | ||||||||||
| 1, 2 | 1,080 (64.3) | 4,418 (63.1) | 5,046 (61.2) | 13,718 (64.0) | 10,194 (65.1) | 34,456 (63.8) | < 0.001 | 10,544 (62.3) | 23,912 (64.5) | < 0.001 |
| 3 | 599 (35.7) | 2,580 (36.9) | 3,203 (38.8) | 7,708 (36.0) | 5,470 (34.9) | 19,560 (36.2) | 6,382 (37.7) | 13,178 (35.5) | ||
| Lymphovascular invasion | ||||||||||
| No | 934 (59.7) | 4,066 (63.1) | 4,296 (62.8) | 12,088 (63.3) | 8,893 (66.1) | 30,277 (63.9) | < 0.001 | 9,296 (62.6) | 20,981 (64.4) | < 0.001 |
| Yes | 631 (40.3) | 2,377 (36.9) | 2,549 (37.2) | 7,015 (36.7) | 4,569 (33.9) | 17,141 (36.1) | 5,557 (37.4) | 11,584 (35.6) | ||
| Body mass index | ||||||||||
| ≤ 25 | 1,007 (51.3) | 4,209 (51.2) | 5,955 (61.0) | 18,325 (73.1) | 14,609 (81.2) | 44,105 (70.0) | < 0.001 | 11,171 (56.0) | 32,934 (76.5) | < 0.001 |
| > 25 | 956 (48.7) | 4,012 (48.8) | 3,813 (39.0) | 6,738 (26.9) | 3,373 (18.8) | 18,892 (30.0) | 8,781 (44.0) | 10,111 (23.5) | ||
| Operation period | ||||||||||
| 1987-2005 | 751 (36.9) | 2,565 (30.4) | 3,860 (38.8) | 7,266 (28.5) | 4,134 (22.7) | 18,576 (29.0) | < 0.001 | 7,176 (35.1) | 11,400 (26.1) | < 0.001 |
| 2006-2010 | 775 (38.1) | 3,317 (39.3) | 3,285 (33.0) | 9,398 (36.9) | 6,201 (34.0) | 22,976 (35.8) | 7,377 (36.1) | 15,599 (35.7) | ||
| 2011-2014 | 509 (25.0) | 2,552 (30.3) | 2,810 (28.2) | 8,800 (34.6) | 7,906 (43.3) | 22,577 (35.2) | 5,871 (28.7) | 16,706 (38.2) | ||
| Operation | ||||||||||
| Lumpectomy | 764 (38.4) | 3,915 (47.3) | 4,889 (50.0) | 14,422 (57.6) | 11,008 (61.5) | 34,998 (55.6) | < 0.001 | 9,568 (47.7) | 25,430 (59.2) | < 0.001 |
| Mastectomy | 1,223 (61.6) | 4,365 (52.7) | 4,889 (50.0) | 10,605 (42.4) | 6,898 (38.5) | 27,980 (44.4) | 10,477 (52.3) | 17,503 (40.8) | ||
| Radiation therapy | ||||||||||
| No | 866 (51.1) | 2,997 (42.7) | 3,529 (41.1) | 7,483 (33.7) | 5,036 (31.1) | 19,911 (35.7) | < 0.001 | 7,392 (42.7) | 12,519 (32.6) | < 0.001 |
| Yes | 830 (48.9) | 4,024 (57.3) | 5,062 (58.9) | 14,706 (66.3) | 11,174 (68.9) | 35,796 (64.3) | 9,916 (57.3) | 25,880 (67.4) | ||
| Chemotherapy | ||||||||||
| No | 594 (33.8) | 1,988 (27.2) | 2,335 (26.0) | 5,703 (24.7) | 5,200 (31.1) | 15,820 (27.4) | < 0.001 | 4,917 (27.2) | 10,903 (27.4) | 0.662 |
| Yes | 1,163 (66.2) | 5,326 (72.8) | 6,640 (74.0) | 17,342 (75.3) | 11,515 (68.9) | 41,986 (72.6) | 13,129 (72.8) | 28,857 (72.6) | ||
| Hormonal therapy | ||||||||||
| No | 514 (46.4) | 2,257 (44.0) | 2,710 (42.8) | 6,368 (36.7) | 4,705 (36.5) | 16,554 (38.6) | < 0.001 | 5,481 (43.6) | 11,073 (36.6) | < 0.001 |
| Yes | 594 (53.6) | 2,876 (56.0) | 3,621 (57.2) | 10,987 (63.3) | 8,201 (63.5) | 26,279 (61.4) | 7,091 (56.4) | 19,188 (63.4) | ||
Table 2.
| Characteristic (age > 50 yr) |
Multivariate analysis |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Univariate analysis |
Biological modela) |
Treatment modelb) |
Combined modelc) |
|||||||||
| Hazard ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value | |
| Education level (high vs. low) | 0.626 | 0.577-0.678 | < 0.001 | 0.691 | 0.615-0.778 | < 0.001 | 0.723 | 0.649-0.804 | < 0.001 | 0.821 | 0.710-0.948 | 0.007 |
| Tumor size (> 2 cm vs. ≤ 2 cm) | 2.663 | 2.458-2.885 | < 0.001 | 2.089 | 1.758-2.483 | < 0.001 | - | - | - | 1.667 | 1.336-2.080 | < 0.001 |
| Node positivity (yes vs. no) | 2.841 | 2.632-3.067 | < 0.001 | 2.275 | 1.946-2.658 | < 0.001 | - | - | - | 2.042 | 1.647-2.533 | < 0.001 |
| Stage (II, III vs. I) | 3.278 | 2.976-3.611 | < 0.001 | 0.789 | 0.618-1.007 | 0.057 | - | - | - | 1.026 | 0.744-1.415 | 0.876 |
| Hormone receptor (positive vs. negative) | 0.719 | 0.664-0.779 | < 0.001 | 0.738 | 0.652-0.836 | < 0.001 | - | - | - | 0.693 | 0.543-0.884 | 0.003 |
| HER2 (positive vs. negative) | 1.139 | 1.033-1.256 | 0.009 | 0.880 | 0.776-0.997 | 0.045 | - | - | - | 0.743 | 0.635-0.869 | < 0.001 |
| Histologic grade (3 vs. 1, 2) | 1.719 | 1.583-1.866 | < 0.001 | 1.462 | 1.296-1.650 | < 0.001 | - | - | - | 1.523 | 1.304-1.778 | < 0.001 |
| Lymphovascular invasion (yes vs. no) | 2.277 | 2.080-2.493 | < 0.001 | 1.464 | 1.297-1.652 | < 0.001 | - | - | - | 1.570 | 1.342-1.837 | < 0.001 |
| Body mass index (> 25 vs. ≤ 25) | 1.117 | 1.036-1.205 | 0.004 | 0.975 | 0.872-1.090 | 0.653 | - | - | - | 1.053 | 0.915-1.212 | 0.471 |
| Operation period | < 0.001 | 0.037 | ||||||||||
| 1987-2005 | Reference | - | Reference | Reference | ||||||||
| 2006-2010 | 0.694 | 0.637-0.755 | < 0.001 | - | - | - | 0.831 | 0.742-0.931 | 0.001 | 0.937 | 0.797-1.101 | 0.427 |
| 2011-2014 | 0.541 | 0.463-0.633 | < 0.001 | - | - | - | 0.639 | 0.522-0.782 | < 0.001 | 0.715 | 0.553-0.926 | 0.011 |
| Operation (mastectomy vs. lumpectomy) | 2.347 | 2.153-2.557 | < 0.001 | - | - | - | 3.716 | 3.291-4.196 | < 0.001 | 1.876 | 1.564-2.249 | < 0.001 |
| Radiation therapy (yes vs. no) | 0.810 | 0.747-0.878 | < 0.001 | - | - | - | 2.304 | 1.986-2.674 | < 0.001 | 1.495 | 1.209-1.849 | < 0.001 |
| Chemotherapy (yes vs. no) | 1.193 | 1.091-1.303 | < 0.001 | - | - | - | 1.037 | 0.904-1.191 | 0.603 | 0.628 | 0.504-0.783 | < 0.001 |
| Hormonal therapy (yes vs. no) | 0.657 | 0.595-0.726 | < 0.001 | - | - | - | 0.710 | 0.628-0.804 | < 0.001 | 0.808 | 0.628-1.039 | 0.096 |
Three models including the biological model, treatment model, and combined model were used for multivariate analyses. CI, confidence interval; HER2, human epidermal growth factor receptor 2.
a) Education level was adjusted with eight factors including tumor size, node positivity, stage, hormonal receptor, HER2, histologic grade, lymphovascular invasion, and body mass index,




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download