1. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer, version 2. Fort Washington, PA: National Comprehensive Cancer Network;2016.
2. Ragaz J, Olivotto IA, Spinelli JJ, Phillips N, Jackson SM, Wilson KS, et al. Locoregional radiation therapy in patients with highrisk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005; 97:116–26.

3. Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol. 2007; 82:247–53.
4. EBCTCG (Early Breast Cancer Trialists' Collaborative Group), McGale P, Taylor C, Correa C, Cutter D, Duane F, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: metaanalysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014; 383:2127–35.
5. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 365:1687–717.
6. De Laurentiis M, Cancello G, D'Agostino D, Giuliano M, Giordano A, Montagna E, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol. 2008; 26:44–53.

7. Mackey JR, Martin M, Pienkowski T, Rolski J, Guastalla JP, Sami A, et al. Adjuvant docetaxel, doxorubicin, and cyclophosphamide in node-positive breast cancer: 10-year follow-up of the phase 3 randomised BCIRG 001 trial. Lancet Oncol. 2013; 14:72–80.

8. Mamounas EP, Bryant J, Lembersky B, Fehrenbacher L, Sedlacek SM, Fisher B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005; 23:3686–96.

9. Sartor CI, Peterson BL, Woolf S, Fitzgerald TJ, Laurie F, Turrisi AJ, et al. Effect of addition of adjuvant paclitaxel on radiotherapy delivery and locoregional control of node-positive breast cancer: cancer and leukemia group B 9344. J Clin Oncol. 2005; 23:30–40.

10. Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Peto R, Davies C, Godwin J, Gray R, Pan HC, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012; 379:432–44.
11. McBride A, Allen P, Woodward W, Kim M, Kuerer HM, Drinka EK, et al. Locoregional recurrence risk for patients with T1,2 breast cancer with 1-3 positive lymph nodes treated with mastectomy and systemic treatment. Int J Radiat Oncol Biol Phys. 2014; 89:392–8.

12. Chang JS, Lee J, Kim KH, Sohn JH, Kim SI, Park BW, et al. Do recent advances in diagnostic and therapeutic procedures negate the benefit of postmastectomy radiotherapy in N1 patients with a low risk of locoregional recurrence? Medicine (Baltimore). 2015; 94:e1259.

13. Taghian A, Jeong JH, Mamounas E, Anderson S, Bryant J, Deutsch M, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol. 2004; 22:4247–54.

14. Yu JI, Park W, Huh SJ, Choi DH, Lim YH, Ahn JS, et al. Determining which patients require irradiation of the supraclavicular nodal area after surgery for N1 breast cancer. Int J Radiat Oncol Biol Phys. 2010; 78:1135–41.

15. Wen G, Zhang JS, Zhang YJ, Zhu YJ, Huang XB, Guan XX. Predictive value of molecular subtyping for locoregional recurrence in early-stage breast cancer with N1 without postmastectomy radiotherapy. J Breast Cancer. 2016; 19:176–84.

16. Moo TA, McMillan R, Lee M, Stempel M, Ho A, Patil S, et al. Impact of molecular subtype on locoregional recurrence in mastectomy patients with T1-T2 breast cancer and 1-3 positive lymph nodes. Ann Surg Oncol. 2014; 21:1569–74.

17. Lai SF, Chen YH, Kuo WH, Lien HC, Wang MY, Lu YS, et al. Locoregional recurrence risk for postmastectomy breast cancer patients with T1-2 and one to three positive lymph nodes receiving modern systemic treatment without radiotherapy. Ann Surg Oncol. 2016; 23:3860–9.

18. Yu JI, Park W, Choi DH, Huh SJ, Nam SJ, Kim SW, et al. Prognostic modeling in pathologic N1 breast cancer without elective nodal irradiation after current standard systemic management. Clin Breast Cancer. 2015; 15:e197.

19. Tendulkar RD, Rehman S, Shukla ME, Reddy CA, Moore H, Budd GT, et al. Impact of postmastectomy radiation on locoregional recurrence in breast cancer patients with 1-3 positive lymph nodes treated with modern systemic therapy. Int J Radiat Oncol Biol Phys. 2012; 83:e577.





 PDF
PDF Citation
Citation Print
Print


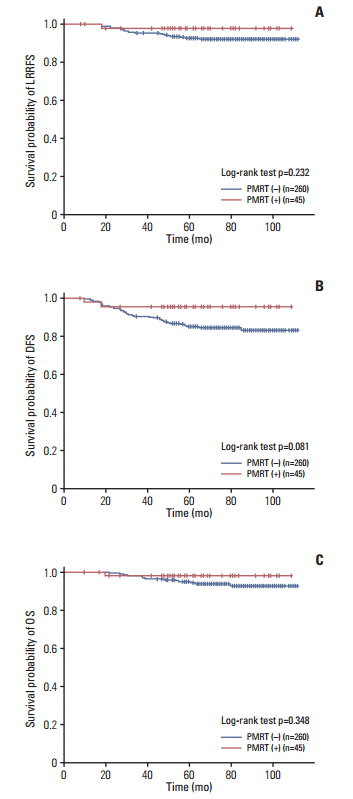
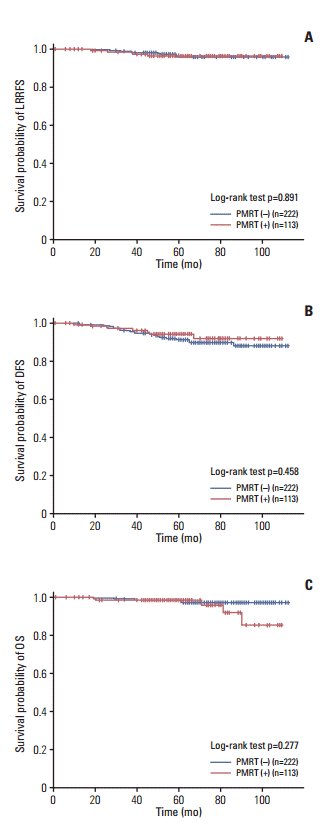
 XML Download
XML Download