Introduction
Materials and Methods
1. Construction of 1615133 TriKE
2. Isolation of inclusion body
3. Refolding and purification
4. Tissue culture
5. Isolation of NK cells and purification
6. Proliferation assay
7. CD107a degranulation assay
8. 51Chromium release cytotoxicity assay
9. Binding/blocking assay
10. Statistical analyses
Results
1. 1615133 production and purification
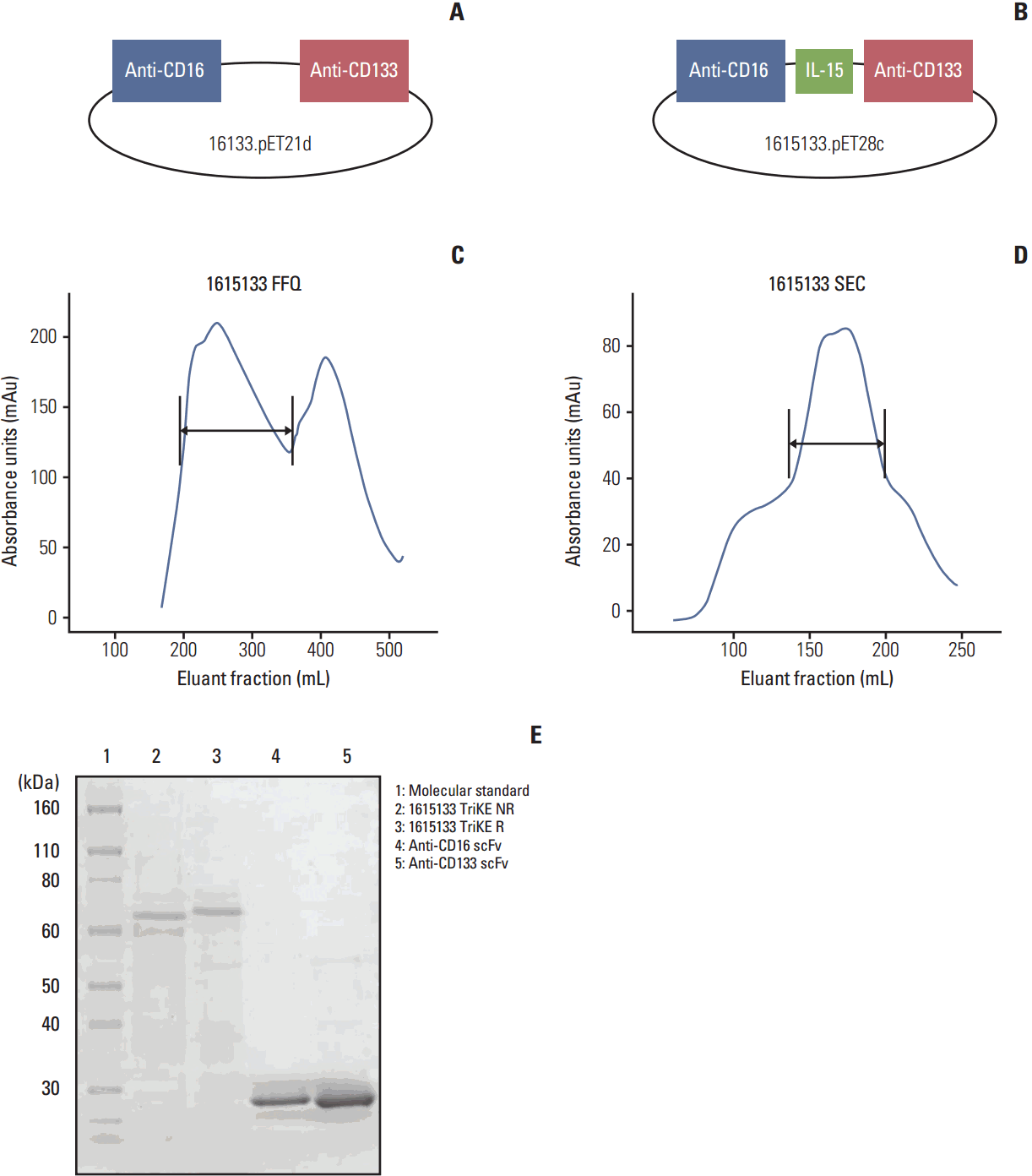 | Fig. 1.Construction and purification. (A) 16133 BiKE platform was modified to produce a NK engager capable of immune expansion. (B) A modified IL-15 crosslinker was incorporated between the VL and VH fragment of anti-CD16 and anti-CD133 scFV forming an IL-15 TriKE (1615133). (C) TriKE trace from the first ion exchange purification column, fast flow sepharose Q (arrow marks appropriate size range). (D) TriKE trace data from the eluent from the second size exclusion column (arrow marks the appropriate size range). (E) The drug is > 90% pure, as determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis. The size is approximately 66,680 Da, as shown by the molecular weight standards in lane 1 (molecular standard). Lane 2 is TriKE non-reduced (1615133 TriKE NR). Lane 3 is TriKE reduced (1615133 TriKE R). For size comparison, lanes 4 and 5 are anti-CD16 and anti-CD133 scFv, respectively. BiKE, bispecific natural killer cell engager; NK, natural killer; IL-15, interleukin 15; scFV, single chain variable fragment; TriKE, trispecific natural killer cell engager. |
2. Activity of the IL-15 moiety
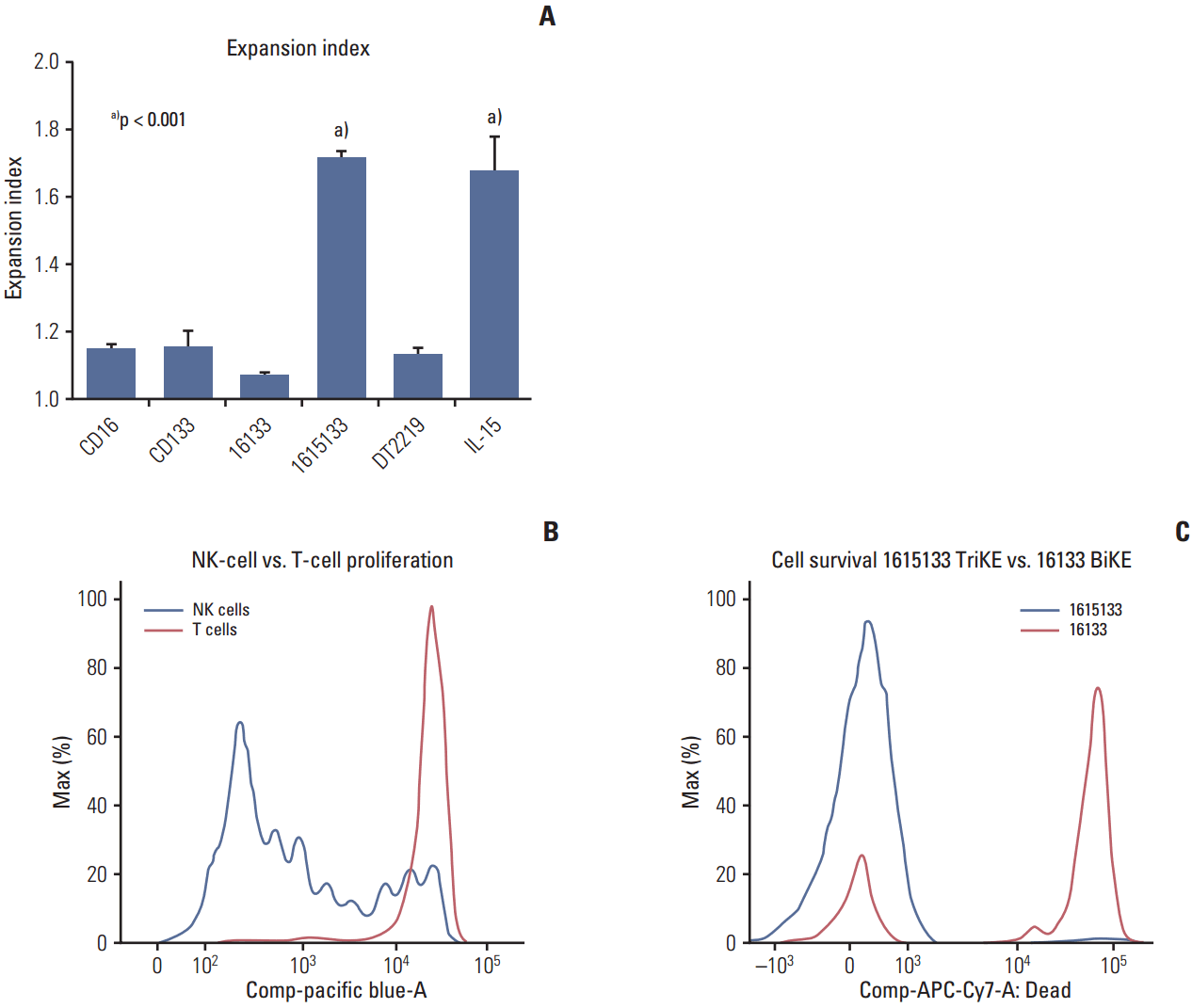 | Fig. 2.Expansion and survival. Purified NK cells were exposed to anti-CD16 scFv (CD16), anti-CD133 scFv (CD133), 16133 BiKE, 1615133 TriKE, DT2219 (a targeted toxin consisting of an anti-CD22 and anti-CD19 scFv linked to a diphtheria toxin), and National Cancer Institute–derived interleukin 15 (IL-15). The graph shows data in both raw histogram form (indicating multiple division cycles) and as a formal calculation of the expansion index. (A) As seen after the evaluation of the expansion index, only TriKE and IL-15 increased proliferation significantly (labeled with the letter a) (n=5). Expansion index was calculated using Flowjo software according to the formula: expansion index=(1–PF)/(1–Dil) (where PF=fraction of the original population divided at least once during the culture period and Dil=percentage of cells in the final population that have divided), for each group [22]. The significance was estimated by one-way ANOVA and presented with standard deviation. (B) Representative histogram illustrates that after gating on NK cells and T cells, a typical proliferative pattern was visible only in NK cells and not in T cells. (C) Purified NK cells were exposed to 1615133 TriKE and 16133 BiKE and incubated for 7 days. The representative histogram illustrates an impressively larger number of live cells with TriKE compared to the construct without the IL-15 moiety. NK, natural killer; scFV, single chain variable fragment; BiKE, bispecific natural killer cell engager; TriKE, trispecific natural killer cell engager; IL-15, interleukin 15. |
3. Activity and specificity of lytic degranulation
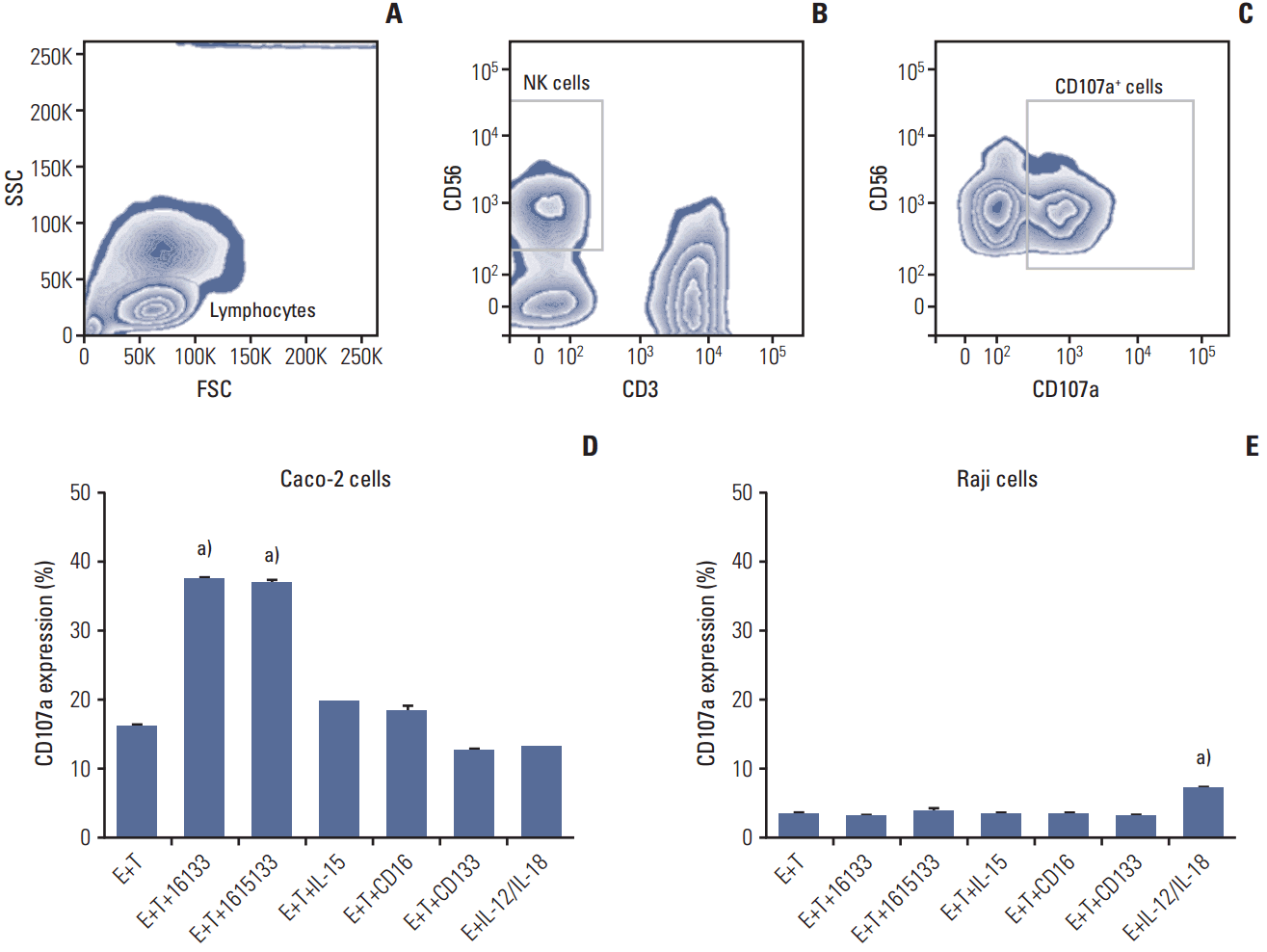 | Fig. 3.Induction of the Degranulation of 1615133 TriKE. (A-C) Gating strategy is shown with the representative Zebraplots of effectors and Caco-2 target cells with 1615133 exposure. (D, E) CD133+ Caco-2 and CD133– Raji cells were exposed to peripheral blood mononuclear cells and 16133 BiKE, 1615133 TriKE, National Cancer Institute–derived IL-15 (IL15), anti-CD16 scFv or anti-CD133 scFv (CD133). The positive control contained peripheral blood mononuclear cells and supraphysiologic concentrations of IL-12 and IL-18 (IL-12/IL-18). With Caco-2 cells the samples with 16133 BiKE and 1615133 TriKE showed significantly higher CD107a expression compared to the controls (noted in graph). For Raji cells, this was only obvious for the IL-12/IL-18 control. The graphs show the pooled data of CD107a expression for each of the groups (n=3). The significance was estimated with one-way ANOVA and presented with the standard deviation. a)p < 0.001. TriKE, trispecific natural killer cell engager; BiKE, bispecific natural killer cell engager; IL, interleukin; SSC, side scatter; FSC, forward scatter; NK, natural killer; E, effector; T, target. |
4. Specificity of binding and activity of the TriKE
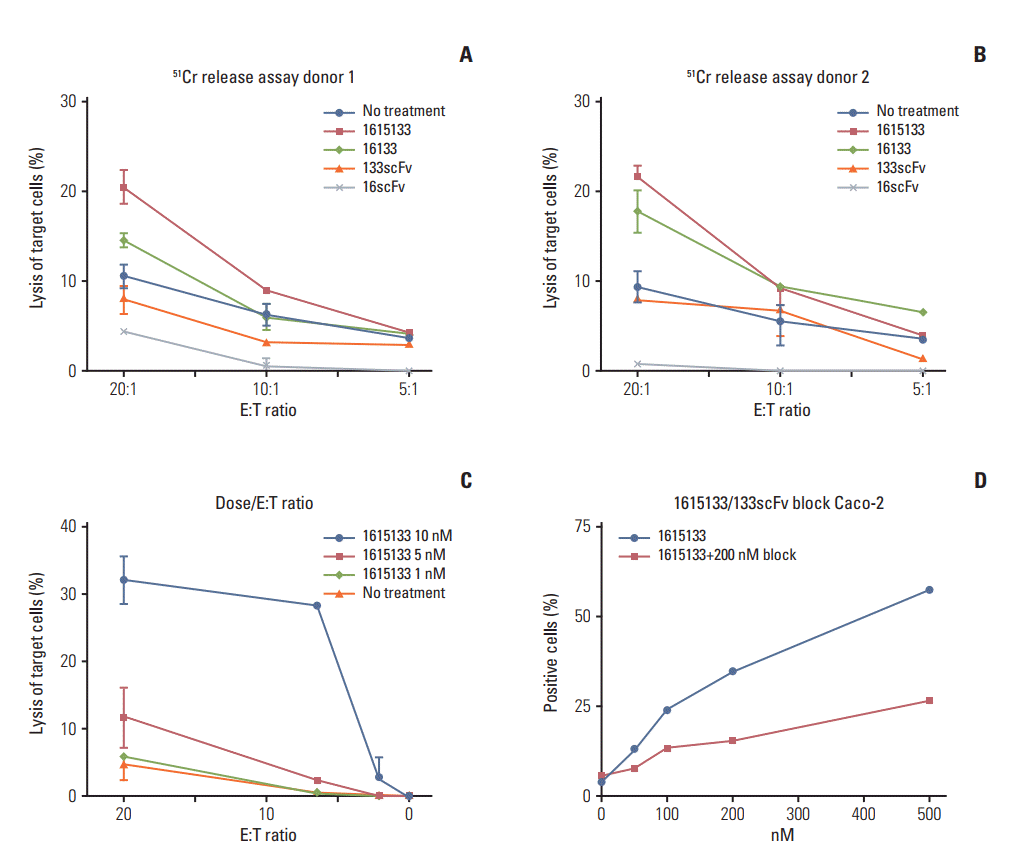 | Fig. 4.
51Chromium release and binding. (A, B) To evaluate the drug activity, 51Cr release assays using two donors were performed. 1615133 TriKE, 16133 BiKE, anti-CD16 scFv (16scFv), and anti-CD133 scFv (133scFv) (30 nM) were cocultured with CD133+ Caco-2 cells and peripheral blood mononuclear cells at the labeled E:T ratios. (C) Peripheral blood mononuclear cells and Caco-2 cells were exposed to different TriKE concentrations (1, 5, and 10 nM) and titered at their E:T ratio (20:1, 6.6:1, 2.2:1, 0.7:1, 0.23:1, and 0.08:1). (D) Fluorescein isothiocyate labeled 1615133 TriKE was incubated at the labeled concentrations with Caco-2 cells. In the same experiment, the same amount of FITC labeled 1615133 was added with 200 nM of unlabeled monomeric CD133 scFv for blocking. TriKE, trispecific natural killer cell engager; BiKE, bispecific natural killer cell engager; scFV, single chain variable fragment; E, effector; T, target. |
5. Ability of IFN-γ induction
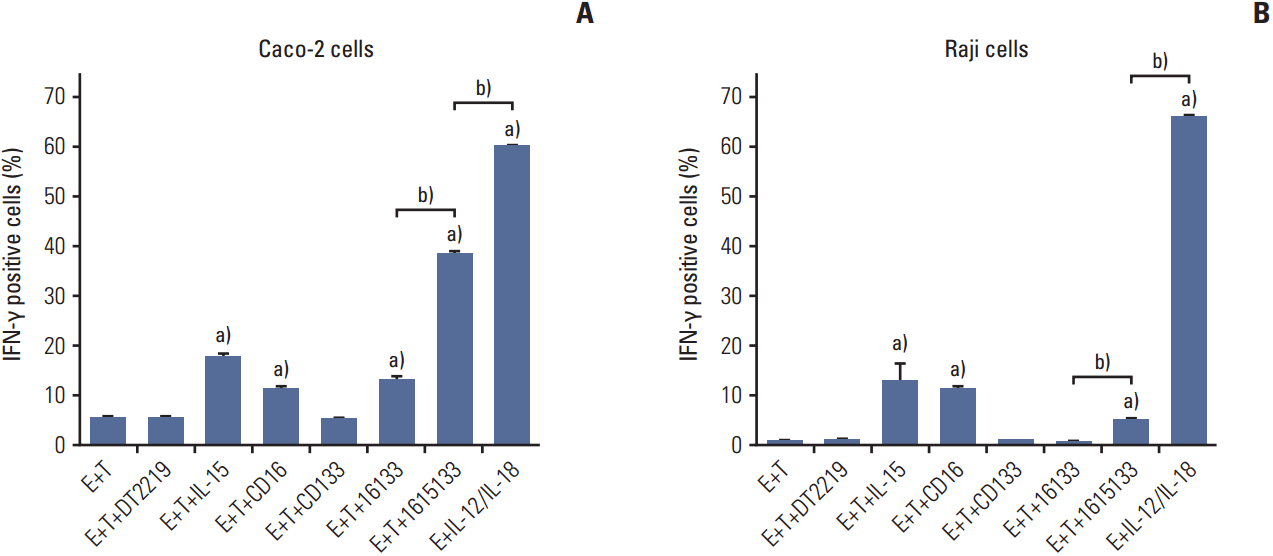 | Fig. 5.Intracellular IFN-γ production. (A) Peripheral blood mononuclear cells and Caco-2 cell targets were exposed to DT2219, National Cancer Institute derived IL-15 (IL-15), anti-CD16 scFv (CD16), anti-CD133 scFv (CD133), 16133 BiKE, 1615133 TriKE (50 nM), or an IL-12 (10 ng/mL) and IL-18 (100 ng/mL) positive control (IL-12/IL-18). The gates were set on CD56+CD3- NK cells. (B) CD133- Raji cells were exposed to the same panel. IFN-γ, interferon γ; IL, interleukin; scFV, single chain variable fragment; BiKE, bispecific natural killer cell engager; TriKE, trispecific natural killer cell engager; NK, natural killer; E, effector; T, target. a)Significance compared to E+T (p < 0.001), b)Significance direct comparison (p < 0.001). |
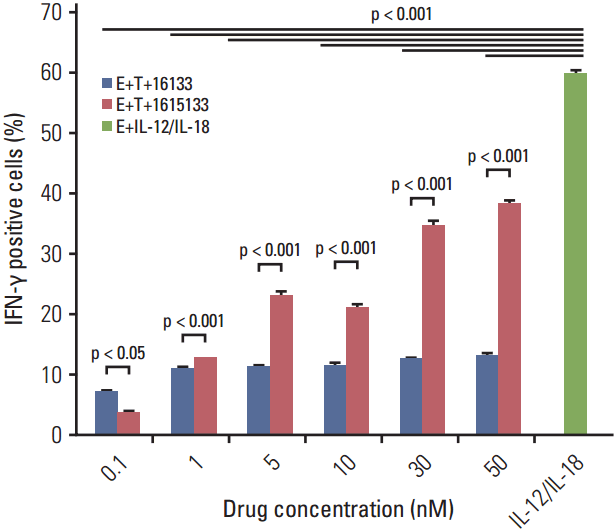 | Fig. 6.Dose dependent IFN-γ production. Caco-2 cells were incubated with peripheral blood mononuclear cells and exposed to labeled doses of the TriKE and BiKE constructs. After gating on NK cells, flow cytometry showed the respective intracellular IFN-γ production. IFN-γ, interferon γ; TriKE, trispecific natural killer cell engager; BiKE, bispecific natural killer cell engager; NK, natural killer; E, effector; T, target; IL, interleukin. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download