1. Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, et al. Endocrine therapy for hormone receptorpositive metastatic breast cancer: American Society of Clinical Oncology Guideline. J Clin Oncol. 2016; 34:3069–103.

2. Taylor CW, Green S, Dalton WS, Martino S, Rector D, Ingle JN, et al. Multicenter randomized clinical trial of goserelin versus surgical ovariectomy in premenopausal patients with receptor-positive metastatic breast cancer: an intergroup study. J Clin Oncol. 1998; 16:994–9.

3. Forward DP, Cheung KL, Jackson L, Robertson JF. Clinical and endocrine data for goserelin plus anastrozole as second-line endocrine therapy for premenopausal advanced breast cancer. Br J Cancer. 2004; 90:590–4.

4. Carlson RW, Theriault R, Schurman CM, Rivera E, Chung CT, Phan SC, et al. Phase II trial of anastrozole plus goserelin in the treatment of hormone receptor-positive, metastatic carcinoma of the breast in premenopausal women. J Clin Oncol. 2010; 28:3917–21.

5. Park IH, Ro J, Lee KS, Kim EA, Kwon Y, Nam BH, et al. Phase II parallel group study showing comparable efficacy between premenopausal metastatic breast cancer patients treated with letrozole plus goserelin and postmenopausal patients treated with letrozole alone as first-line hormone therapy. J Clin Oncol. 2010; 28:2705–11.

6. Yao S, Xu B, Li Q, Zhang P, Yuan P, Wang J, et al. Goserelin plus letrozole as first- or second-line hormonal treatment in premenopausal patients with advanced breast cancer. Endocr J. 2011; 58:509–16.

7. Nishimura R, Anan K, Yamamoto Y, Higaki K, Tanaka M, Shibuta K, et al. Efficacy of goserelin plus anastrozole in premenopausal women with advanced or recurrent breast cancer refractory to an LH-RH analogue with tamoxifen: results of the JMTO BC08-01 phase II trial. Oncol Rep. 2013; 29:1707–13.

8. Wang J, Xu B, Yuan P, Ma F, Li Q, Zhang P, et al. Phase II trial of goserelin and exemestane combination therapy in premenopausal women with locally advanced or metastatic breast cancer. Medicine (Baltimore). 2015; 94:e1006.

9. Schmid P, Untch M, Kosse V, Bondar G, Vassiljev L, Tarutinov V, et al. Leuprorelin acetate every-3-months depot versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant treatment in premenopausal patients with node-positive breast cancer: the TABLE study. J Clin Oncol. 2007; 25:2509–15.

10. Bellet M, Gray KP, Francis PA, Lang I, Ciruelos E, Lluch A, et al. Twelve-month estrogen levels in premenopausal women with hormone receptor-positive breast cancer receiving adjuvant triptorelin plus exemestane or tamoxifen in the Suppression of Ovarian Function Trial (SOFT): The SOFT-EST Substudy. J Clin Oncol. 2016; 34:1584–93.

11. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010; 28:2784–95.

12. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013; 31:3997–4013.

13. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–47.
14. Kaufmann M, Jonat W, Kleeberg U, Eiermann W, Janicke F, Hilfrich J, et al. Goserelin, a depot gonadotrophin-releasing hormone agonist in the treatment of premenopausal patients with metastatic breast cancer. German Zoladex Trial Group. J Clin Oncol. 1989; 7:1113–9.

15. Blamey RW, Jonat W, Kaufmann M, Bianco AR, Namer M. Goserelin depot in the treatment of premenopausal advanced breast cancer. Eur J Cancer. 1992; 28A:810–4.

16. Boccardo F, Rubagotti A, Perrotta A, Amoroso D, Balestrero M, De Matteis A, et al. Ovarian ablation versus goserelin with or without tamoxifen in pre-perimenopausal patients with advanced breast cancer: results of a multicentric Italian study. Ann Oncol. 1994; 5:337–42.

17. Pagani O, Regan MM, Walley BA, Fleming GF, Colleoni M, Lang I, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014; 371:107–18.
18. Francis PA, Regan MM, Fleming GF, Lang I, Ciruelos E, Bellet M, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015; 372:436–46.

19. Gnant M, Mlineritsch B, Stoeger H, Luschin-Ebengreuth G, Knauer M, Moik M, et al. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann Oncol. 2015; 26:313–20.

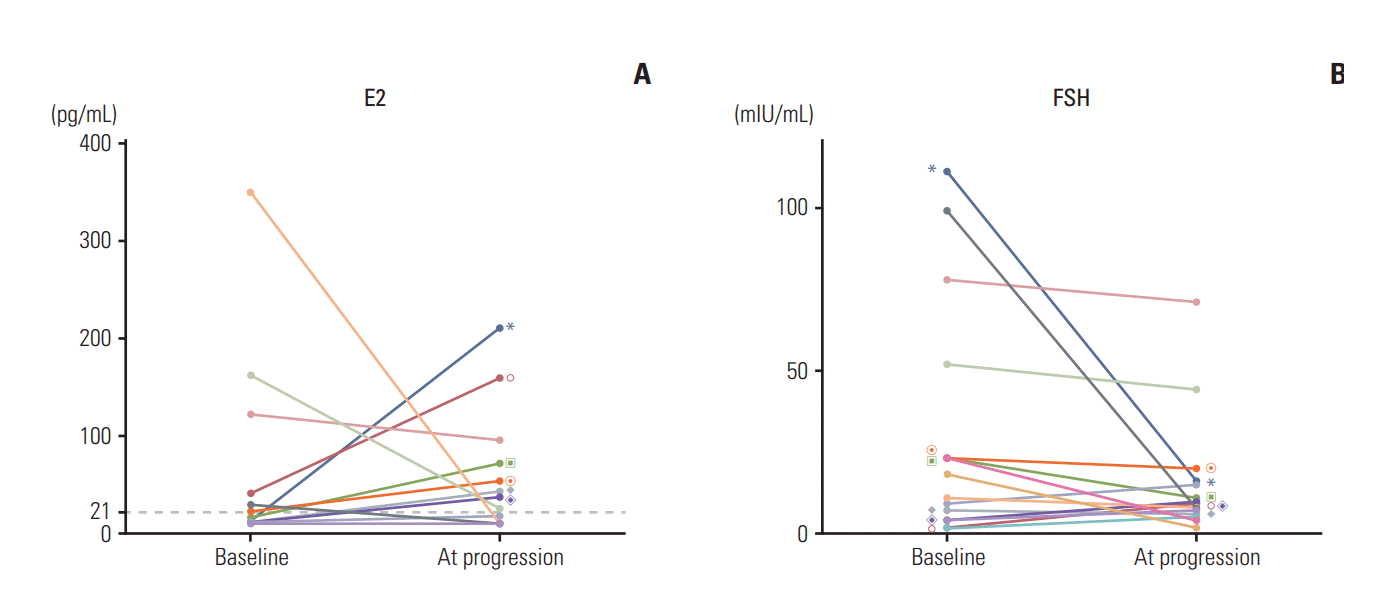
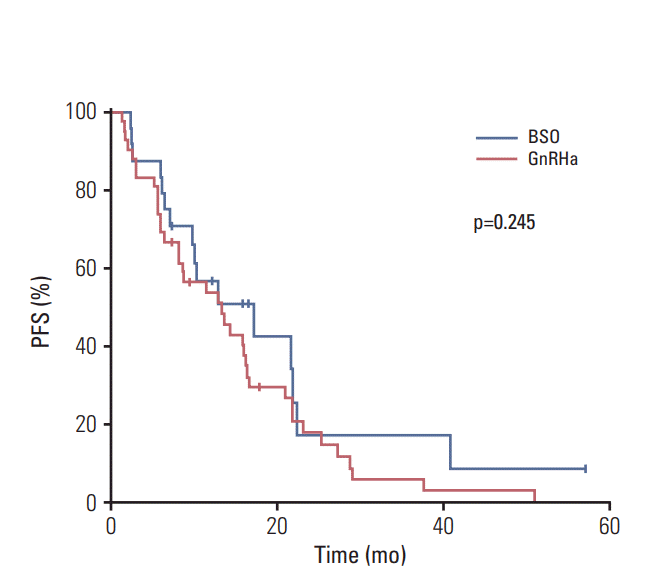
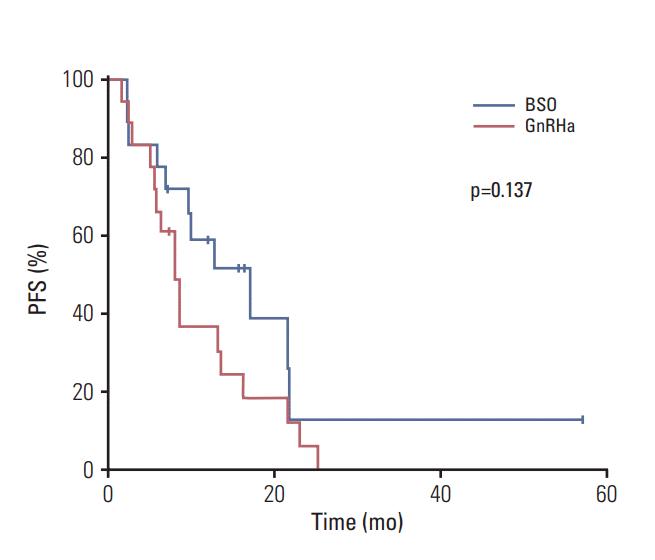
20. Papakonstantinou A, Foukakis T, Rodriguez-Wallberg KA, Bergh J. Is estradiol monitoring necessary in women receiving ovarian suppression for breast cancer? J Clin Oncol. 2016; 34:1573–9.

21. Ellis MJ, Llombart-Cussac A, Feltl D, Dewar JA, Jasiowka M, Hewson N, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: overall survival analysis From the Phase II FIRST Study. J Clin Oncol. 2015; 33:3781–7.

22. Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormonereceptor-positive advanced breast cancer. N Engl J Med. 2012; 366:520–9.

23. Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016; 17:425–39.

24. Finn RS, Martin M, Rugo HS, Jones SE, Im SA, Gelmon KA, et al. PALOMA-2: primary results from a phase III trial of palbociclib (P) with letrozole (L) compared with letrozole alone in postmenopausal women with ER+/HER2–advanced breast cancer (ABC). J Clin Oncol. 2016; 34(Suppl):Abstr 507.
25. Cho YJ, Kim ML, Lee SY, Lee HS, Kim JM, Joo KY. Laparoendoscopic single-site surgery (LESS) versus conventional laparoscopic surgery for adnexal preservation: a randomized controlled study. Int J Womens Health. 2012; 4:85–91.

26. Angioni S, Pontis A, Sedda F, Zampetoglou T, Cela V, Mereu L, et al. Single-port versus conventional multiport access prophylactic laparoscopic bilateral salpingo-oophorectomy in high-risk patients for ovarian cancer: a comparison of surgical outcomes. Onco Targets Ther. 2015; 8:1575–80.

27. Hsieh AH, Kichenadasse G, Vatandoust S, Roy A, Sukumaran S, Karapetis CS, et al. Goserelin toxicities and preferences for ovarian suppression method in pre-menopausal women with breast cancer. Intern Med J. 2016; 46:1153–9.

28. Hagemann AR, Zighelboim I, Odibo AO, Rader JS, Mutch DG, Powell MA. Cost-benefit of laparoscopic versus medical ovarian suppression in premenopausal breast cancer. Breast J. 2011; 17:103–5.

29. Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype: ACOSOG Z1031. J Clin Oncol. 2011; 29:2342–9.
30. Anderson H, Hills M, Zabaglo L, A'Hern R, Leary AF, Haynes BP, et al. Relationship between estrogen receptor, progesterone receptor, HER-2 and Ki67 expression and efficacy of aromatase inhibitors in advanced breast cancer. Ann Oncol. 2011; 22:1770–6.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download