Introduction
Materials and Methods
1. Patients
2. Radiotherapy
3. Clinical factors
4. Outcome evaluation
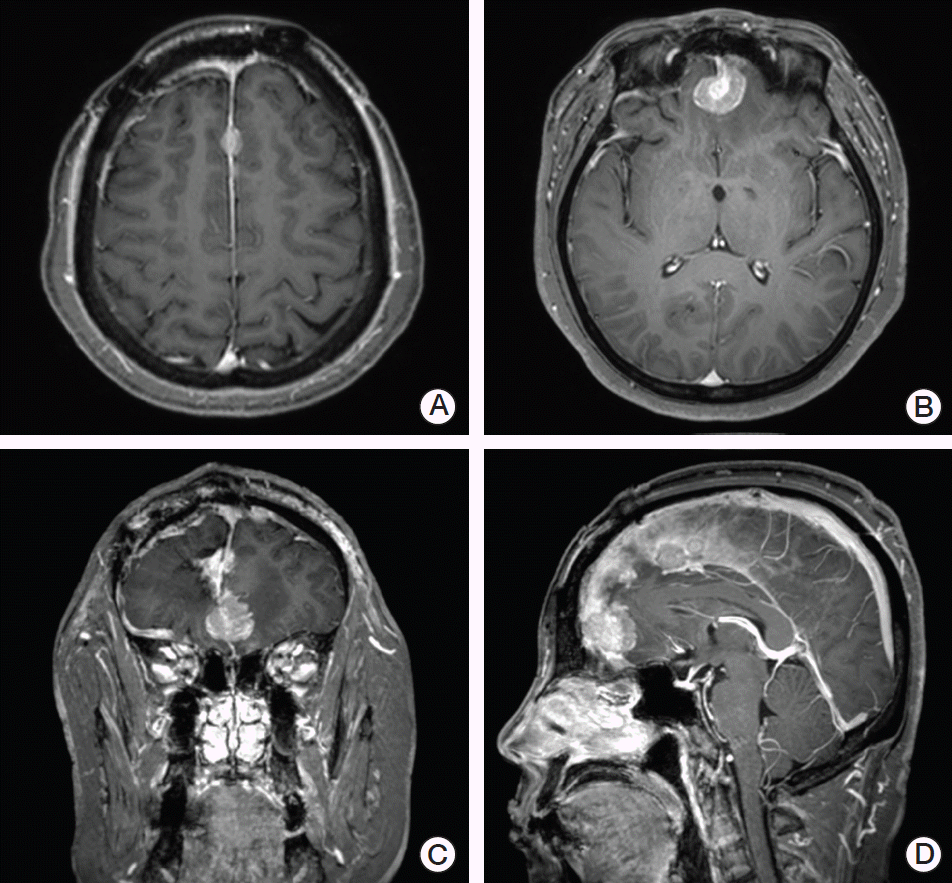
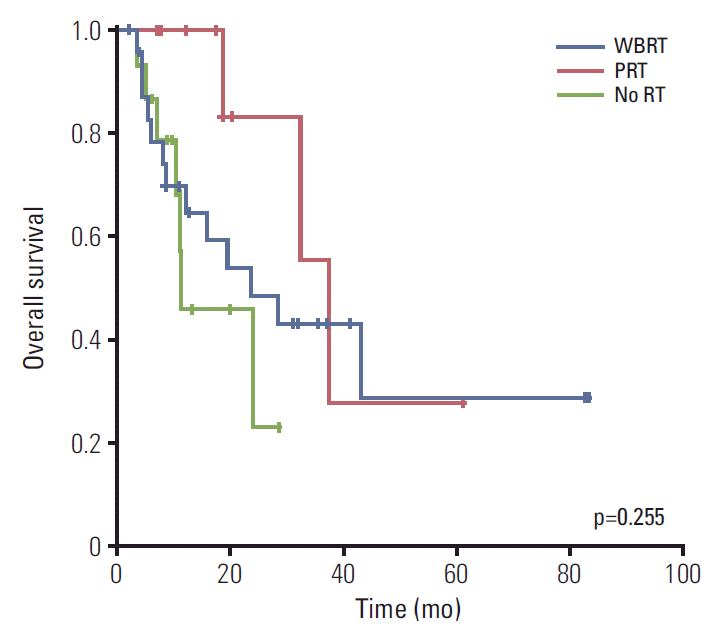
Fig. 1.
5. Statistical analysis
Results
1. Patient, tumor, and treatment characteristics
Table 1.
| Characteristic | All (n=51) | WBRT (n=24) | PRT (n=10) | No RT (n=17) | p-value |
|---|---|---|---|---|---|
| Age, median (range, yr) | 48 (34-75) | 46 (34-68) | 45 (34-68) | 60 (34-75) | 0.805a) |
| Duration between brain metastasis and breast cancer diagnosis, median (range, mo) | 35.1 (5.0-148.0) | 29.9 (7.8-148.0) | 32.4 (9.8-67.5) | 41 (5.0-115.9) | 0.835a) |
| Extracranial disease status | |||||
| None or stable | 35 (69) | 15 (63) | 10 (100) | 10 (59) | 0.056b) |
| Progressive | 16 (31) | 9 (38) | 0 | 7 (41) | |
| Biological subtype of primary tumor | |||||
| ER+ and/or PR+, HER2− | 7 (14) | 3 (13) | 0 | 4 (24) | 0.556b) |
| HER2-positive | 31 (60) | 14 (58) | 7 (70) | 10 (59) | |
| Triple-negative | 13 (26) | 7 (29) | 3 (30) | 3 (18) | |
| ECOG performance status | |||||
| 0-1 | 47 (92) | 22 (92) | 9 (90) | 16 (94) | > 0.990b) |
| 2 | 4 (8) | 2 (8) | 1 (10) | 1 (6) | |
| Breast-GPA | |||||
| 3.5-4.0 | 13 (26) | 5 (21) | 5 (50) | 3 (18) | 0.314b) |
| 2.5-3.0 | 21 (41) | 9 (38) | 2 (20) | 10 (56) | |
| 1.5-2.0 | 15 (29) | 8 (33) | 3 (30) | 4 (24) | |
| 0.5-1.0 | 2 (4) | 2 (8) | 0 | 0 | |
| No. of brain metastases | |||||
| 1 | 36 (71) | 14 (58) | 7 (70) | 15 (88) | 0.117b) |
| 2-3 | 15 (29) | 10 (42) | 3 (30) | 2 (12) | |
| Size, median (range, cm) | 3.5 (1.0-8.0) | 3.6 (1.6-6.0) | 3.5 (2.0-6.0) | 2.9 (1.0-8.0) | 0.518a) |
| Adjacent to CSF flowc) | |||||
| No | 10 (20) | 4 (17) | 1 (10) | 5 (29) | 0.479b) |
| Yes | 40 (80) | 19 (83) | 9 (90) | 12 (71) | |
| Extent of resection | |||||
| GTR | 40 (78) | 18 (75) | 7 (70) | 15 (88) | 0.447b) |
| STR | 11 (22) | 6 (25) | 3 (30) | 2 (12) | |
| HRT after brain surgery | |||||
| Yes | 6 (12) | 3 (6) | 2 (20) | 1 (6) | 0.549b) |
| No | 45 (88) | 21 (88) | 8 (80) | 16 (94) | |
| Systemic treatment after brain surgery and before the development of LMCDM | |||||
| No | 21 (42) | 7 (29) | 4 (40) | 10 (59) | 0.265b) |
| Targeted Tx±CTx | 13 (26) | 6 (25) | 4 (40) | 3 (18) | |
| CTx | 17 (33) | 11 (46) | 2 (20) | 4 (24) | |
| Systemic treatment after brain surgery (at any time) | |||||
| No | 16 (31) | 7 (29) | 1 (10) | 8 (47) | 0.291b) |
| CTx with targeted Tx | 15 (29) | 6 (25) | 5 (50) | 4 (24) | |
| CTx without targeted Tx | 20 (39) | 11 (46) | 4 (40) | 5 (29) |
Values are presented as number (%). WBRT, whole brain radiotherapy; PRT, partial radiotherapy; RT, radiotherapy; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; ECOG, Eastern Cooperative Oncology Group; Breast-GPA, breast specific graded prognostic assessment score; CSF, cerebrospinal fluid; GTR, gross total resection; STR, subtotal resection; HRT, hormone therapy; Tx, therapy; CTx, chemotherapy.
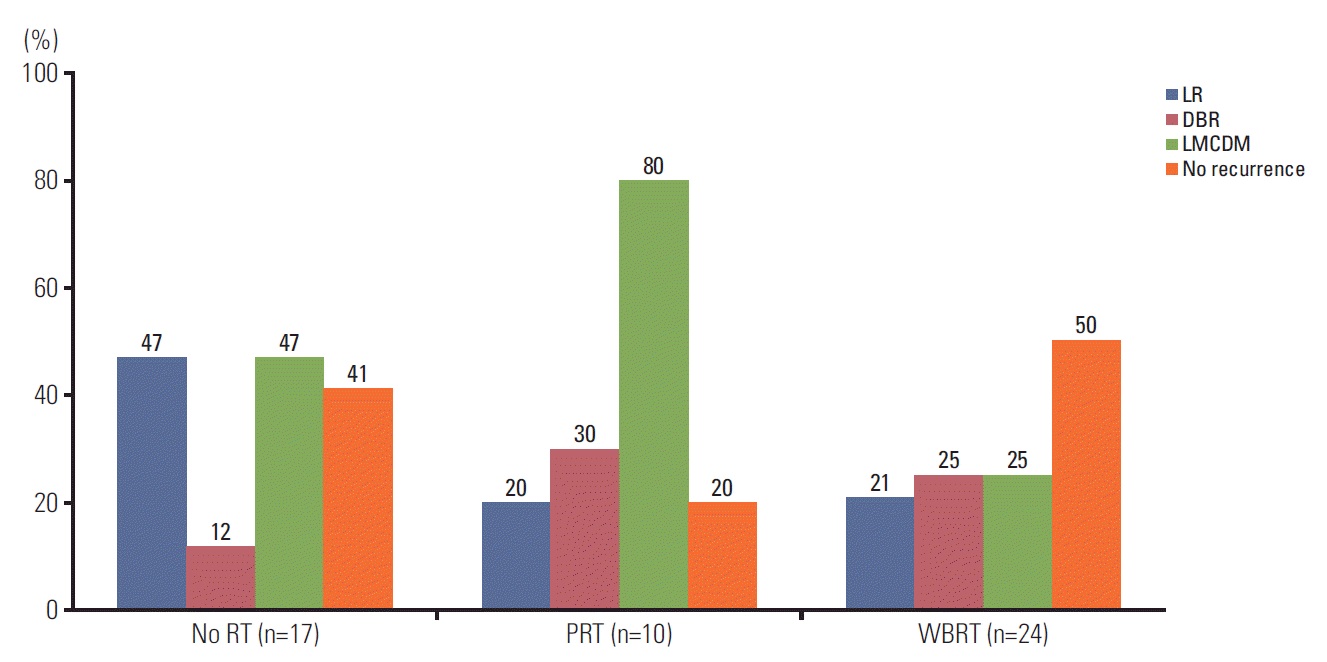
2. Intracranial recurrence rate and pattern of failure
Fig. 2.
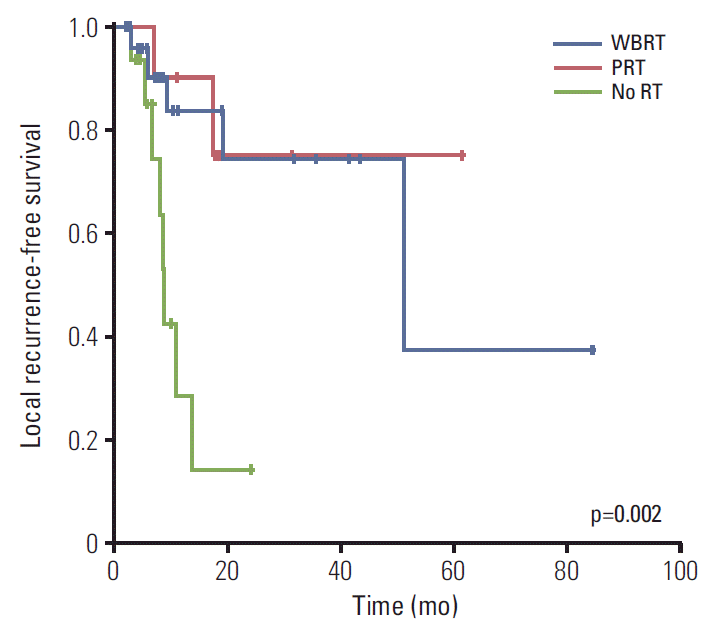
3. Leptomeningeal carcinomatosis or dural metastasis
Fig. 3.
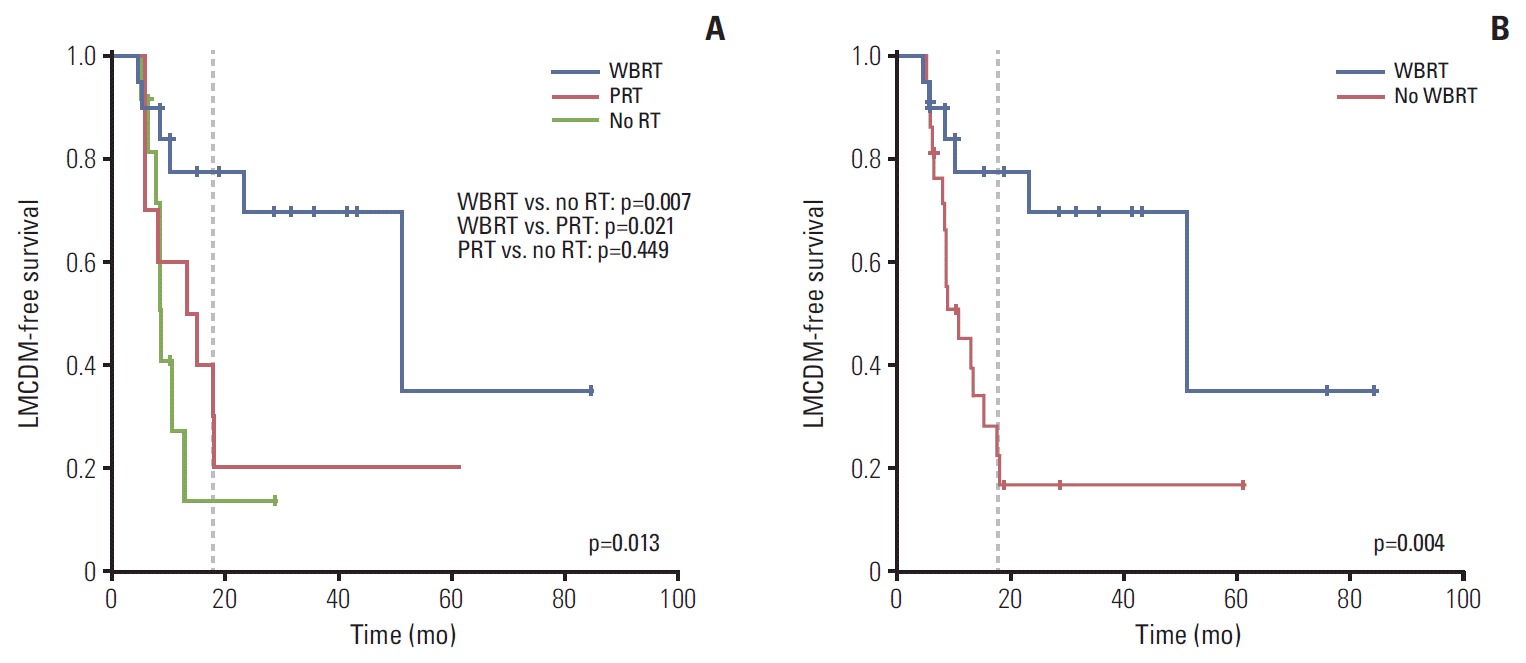
Table 2.
| Characteristic | No. of patients |
Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|---|
| 18-Month LMCDM-FS (%) | p-valuea) | Hazard ratiob) | 95% CI | p-valuec) | ||
| Extracranial disease status | ||||||
| None or stable | 35 | 42.1 | 0.113 | |||
| Progressive | 16 | 64.8 | ||||
| Biological subtype of primary tumor | ||||||
| ER+ and/or PR+, HER2− | 7 | 53.6 | 0.882 | |||
| HER2-positive | 19 | 44.1 | ||||
| Triple negative | 13 | 58.3 | ||||
| No. of brain metastases | ||||||
| 1 | 36 | 45.1 | 0.191 | |||
| 2-3 | 15 | 60.0 | ||||
| Size (cm) | ||||||
| < 3.5 | 23 | 51.3 | 0.906 | |||
| ≥ 3.5 | 27 | 41.1 | ||||
| Adjacent to CSF flowd) | ||||||
| No | 10 | 80.0 | 0.050 | 1.0 | - | - |
| Yes | 40 | 40.3 | 2.3 | 0.4-13.5 | 0.456 | |
| Extent of resection | ||||||
| GTR | 40 | 42.6 | 0.120 | |||
| STR | 11 | 71.4 | ||||
| Systemic treatment after brain surgery and before the development of LMCDM | ||||||
| No | 21 | 8.0 | < 0.001 | 1.0 | - | - |
| Targeted Tx±CTx | 13 | 69.8 | 0.1 | 0.0-0.3 | < 0.001 | |
| CTx | 17 | 67.5 | 0.1 | 0.0-0.3 | < 0.001 | |
| Postoperative RT | ||||||
| WBRT | 24 | 77.5 | 0.013 | 1.0 | - | - |
| PRT | 10 | 30.0 | 5.2 | 1.9-14.8 | 0.009 | |
| No RT | 17 | 13.6 | 2.7 | 0.9-7.9 | 0.122 | |
| Postoperative WBRT | ||||||
| Yes | 24 | 77.5 | 0.004 | |||
| No | 27 | 22.6 | ||||
| WBRT dose−fractionation | ||||||
| 25 Gy/10 fx | 9 | 65.6 | 0.945 | |||
| 30 Gy/12 fx | 6 | 100.0 | ||||
| 30 Gy/10 fx | 9 | 71.4 | ||||
LMCDM-FS, leptomeningeal carcinomatosis or dural metastasis–free survival; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; CSF, cerebrospinal fluid; GTR, gross total resection; STR, subtotal resection; Tx, therapy; CTx, chemotherapy; RT, radiotherapy; WBRT, whole brain radiotherapy; PRT, partial radiotherapy; fx, fractions.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download