1. He JH, Zong YS, Luo RZ, Liang XM, Wu QL, Liang YJ. Clinicopathological characteristics of primary nasopharyngeal adenocarcinoma. Ai Zheng. 2003; 22:753–7.
2. McGuire LJ, Lee JC. The histopathologic diagnosis of nasopharyngeal carcinoma. Ear Nose Throat J. 1990; 69:229–36.
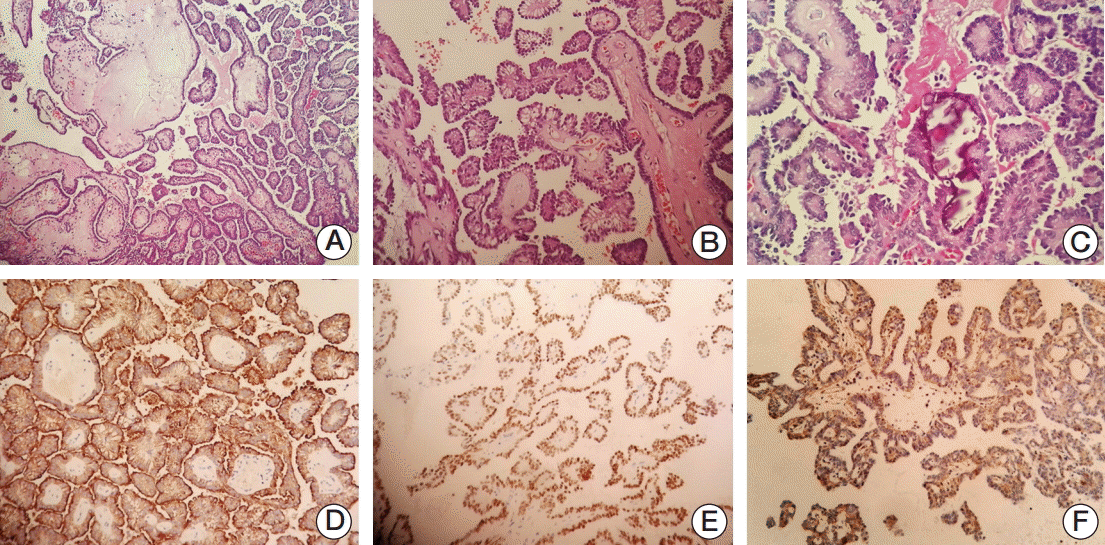
3. Carrizo F, Luna MA. Thyroid transcription factor-1 expression in thyroid-like nasopharyngeal papillary adenocarcinoma: report of 2 cases. Ann Diagn Pathol. 2005; 9:189–92.

4. Wu PY, Huang CC, Chen HK, Chien CY. Adult thyroid-like low-grade nasopharyngeal papillary adenocarcinoma with thyroid transcription factor-1 expression. Otolaryngol Head Neck Surg. 2007; 137:837–8.

5. Fu CH, Chang KP, Ueng SH, Wu CC, Hao SP. Primary thyroid-like papillary adenocarcinoma of the nasopharynx. Auris Nasus Larynx. 2008; 35:579–82.

6. Bansal A, Pradeep KE, Gumparthy KP. An unusual case of low-grade tubulopapillary adenocarcinoma of the sinonasal tract. World J Surg Oncol. 2008; 6:54.

7. Ohe C, Sakaida N, Tadokoro C, Fukui H, Asako M, Tomoda K, et al. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: report of two cases. Pathol Int. 2010; 60:107–11.

8. Sillings CN, Weathers DR, Delgaudio JM. Thyroid-like papillary adenocarcinoma of the nasopharynx: a case report in a 19-year-old male. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 110:e25.

9. Petersson F, Pang B, Loke D, Hao L, Yan B. Biphasic low-grade nasopharyngeal papillary adenocarcinoma with a prominent spindle cell component: report of a case localized to the posterior nasal septum. Head Neck Pathol. 2011; 5:306–13.

10. Huang CH, Chang YL, Wang CP, Wu HP. Positive immunostaining of thyroid transcription factor-1 in primary nasopharyngeal papillary adenocarcinoma. J Formos Med Assoc. 2015; 114:473–4.

11. Ozer S, Kayahan B, Cabbarzade C, Bugdayci M, Kosemehmetoglu K, Yucel OT. Thyroid-like papillary adenocarcinoma of the nasopharynx with focal thyroglobulin expression. Pathology. 2013; 45:622–4.

12. Oishi N, Kondo T, Nakazawa T, Mochizuki K, Kasai K, Inoue T, et al. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: case report and literature review. Pathol Res Pract. 2014; 210:1142–5.

13. Ozturk K, Midilli R, Veral A, Ertan Y, Karci B. Primary thyroid-like papillary adenocarcinoma of the nasal septum: a case report. Ear Nose Throat J. 2015; 94:E19–21.

14. Wu R, Liu H. Clinicopathological features of low-grade nasopharyngeal papillary adenocarcinoma. Zhonghua Bing Li Xue Za Zhi. 2014; 43:613–7.
15. Zhang X, Xu X, Qian K, Liu Y, Wang L, Li M, et al. Low-grade thyroid-like papillary of the nasopharynx with squamous differentiation: report of a case. Lin Chuang Yu Shi Yan Bing Li Xue Za Zhi. 2015; 31:1199–200.
16. Kang Z, Ou Y, Yao L, Qu L, Zheng Z, Yu Y. Low-grade nasopharyngeal papillary adenocarcinoma: report of two cases and literature review. Lin Chuang Yu Shi Yan Bing Li Xue Za Zhi. 2015; 31:1165–7.
17. Li JF, Ye Q, Hong B, Gao X, Xu KL. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: report of a case. Zhonghua Bing Li Xue Za Zhi. 2011; 40:638–9.
18. Han S, Yang F, Yuan C, Zhang R. Clinicopathological observation of 1 cases of low grade nasopharyngeal papillary adenocarcinoma. Lin Chuang Yu Shi Yan Bing Li Xue Za Zhi. 2013; 29:328–30.
19. Chen Z, Zhuo M, Zheng X. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: one case report. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014; 28:1266–7.
20. Wenig BM, Hyams VJ, Heffner DK. Nasopharyngeal papillary adenocarcinoma: a clinicopathologic study of a low-grade carcinoma. Am J Surg Pathol. 1988; 12:946–53.
21. Thompson L. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Ear Nose Throat J. 2006; 85:74.

22. Bingle CD. Thyroid transcription factor-1. Int J Biochem Cell Biol. 1997; 29:1471–3.

23. Katoh R, Kawaoi A, Miyagi E, Li X, Suzuki K, Nakamura Y, et al. Thyroid transcription factor-1 in normal, hyperplastic, and neoplastic follicular thyroid cells examined by immunohistochemistry and nonradioactive in situ hybridization. Mod Pathol. 2000; 13:570–6.

24. Ordonez NG. Value of thyroid transcription factor-1 immunostaining in tumor diagnosis: a review and update. Appl Immunohistochem Mol Morphol. 2012; 20:429–44.
25. Imamura Y. CD15 (C3D-1) immunoreactivity in normal, benign and malignant thyroid lesions. Appl Immunohistochem. 1998; 6:181–6.

26. Ortiz-Rey JA, Alvarez C, San Miguel P, Iglesias B, Anton I. Expression of CDX2, cytokeratins 7 and 20 in sinonasal intestinal-type adenocarcinoma. Appl Immunohistochem Mol Morphol. 2005; 13:142–6.

27. Wang CP, Chang YL, Chen CT, Yang TH, Lou PJ. Photodynamic therapy with topical 5-aminolevulinic acid as a post-operative adjuvant therapy for an incompletely resected primary nasopharyngeal papillary adenocarcinoma: a case report. Lasers Surg Med. 2006; 38:435–8.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download