Abstract
Purpose
Eribulin mesilate was approved for the treatment of patients with locally advanced or metastatic breast cancer (MBC), who had received at least two chemotherapeutic regimens, including anthracycline and taxane. On the other hand, the efficacy and safety information of eribulin in Korean patients is limited by the lack of clinical trials.
Materials and Methods
In this multicenter, open-label, single-arm, phase IV study, locally advanced or MBC patients were enrolled between June 2013 and April 2014 from 14 centers in Korea. One point four mg/m2 dose of eribulin was administered on days 1 and 8 of every 21 days. The primary endpoint was the frequency and intensity of the treatment emergent adverse event. The secondary endpoint was the disease control rate, which included the rate of complete responses, partial responses, and stable disease.
Results
A total of 101 patients received at least one dose of eribulin and were included in the safety set. The patients received a total of 543 treatment cycles, with a median of three cycles (range, 1 to 31 cycles). The most common adverse event was neutropenia (91.1% of patients, 48.3% of cycles). The frequent non-hematological adverse events included alopecia, decrease in appetite, fatigue/asthenia, and myalgia/arthralgia. The peripheral neuropathy of any grade occurred in 27 patients (26.7%), including grade 3 in two patients. Disease control rate was 52.7% and 51.3% of patients in the full analysis set and per-protocol set, respectively.
Breast cancer is the most common cancer and the leading cause of cancer death among women worldwide [1]. Despite the appropriate treatment, approximately 30% of patients with breast cancer eventually progress to metastatic disease [2]. Although there has been considerable progress in the treatment of advanced or metastatic breast cancer (MBC) [3], novel therapeutic approaches are still needed to improve the clinical outcomes for patients with the disease.
Eribulin mesilate, a non-taxane microtubule stabilizer, is currently approved for the treatment of patients with locally advanced or MBC, who have progressed after at least two chemotherapeutic regimens, including anthracycline and taxane. The phase III EMBRACE study demonstrated the overall survival benefit of eribulin over the treatment of physician’s choice by a median of 2.5 months [4]. Although another phase III study failed to show significant improvements in the overall survival with eribulin versus capecitabine [5], a statistically significant survival benefit was observed in the data from a pooled analysis of the two phase III studies in patients with locally advanced or MBC [6]. In addition, a phase II study of eribulin in Japanese patients revealed comparable efficacy and safety [7].
Nevertheless, the toxicity profile and efficacy can differ according to the population and ethnic differences, even if the agent does not perform as a pharmacogenetics drug by polymorphism [8-10]. In addition, the effective dose levels of the anti-cancer drugs can be suboptimal for some populations, particularly in Asian patients [11]. Therefore, eribulin mesilate needs to be validated for Korean patients. This paper reports the results from a phase IV study that evaluated the efficacy and safety of eribulin in Korean patients with locally advanced or MBC.
Female patients with locally advanced or MBC were eligible for the study. The other key inclusion criteria included the following: patients who had received two to five prior chemotherapy regimens for advanced and/or metastatic disease, and prior therapy that included anthracycline and taxane in either the adjuvant or metastatic setting; patients must have been refractory to the most recent chemotherapy on or within the previous 6 months; patients with adequate bone marrow, liver, and renal function; and Eastern Cooperative Oncology Group performance status of 0 to 2.
The key exclusion criteria included the following: patients who had received chemotherapy, radiation, biologics, immunotherapy, or hormonal therapy within the previous 3 weeks before treatment (palliative radiation was allowed); patients with known brain metastases unless treated and stable; patients who had participated in other clinical trials within 4 weeks before screening; patients who had previously received eribulin.
A multicenter, open-label, single-arm, phase IV clinical trial was conducted in accordance with the declaration of Helsinki and approved by the institutional review board of each center. All procedures were performed according to local guidelines. Patients provided written informed consent before administration of the study drug (Clinicaltrial.gov identifier: NCT01961544).
The patients received intravenous eribulin 1.4 mg/m2 over 2-5 minutes on days 1 and 8 of every 21 days. The administration of eribulin had to be delayed in cases of an absolute neutrophil count of less than 1×109/L, platelet count of less than 75×109/L, and grade 3 or 4 nonhematological toxicities. The dose of eribulin was reduced to 1.1 mg/m2 if predefined hematological toxicity or grade 3/4 nonhematological toxicity occurred after eribulin administration. If any adverse events recurred despite the previous dose reduction, the daily dose of eribulin was reduced further to 0.7 mg/m2 and discontinued if the dose reduction was less than 0.7 mg/m2. Once the dose was reduced, it could not be increased at a later date.
The primary endpoint was the frequency and intensity of the treatment emergent adverse events (TEAE). The TEAE were defined as the adverse event started or exacerbated on or after the first dose of the study drug. The intensity of the adverse events was classified by National Cancer Institute Common Terminology Criteria for Adverse Events ver. 4.03. The causal relationship between the adverse event and study drug was also evaluated.
Secondary endpoint was the disease control rate (DCR), which included the rate of complete responses (CRs), partial responses (PRs), and stable disease (SD). The tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors ver. 1.1 [12]. The tumor response assessment was performed every 9 weeks (±1 week) and at the end of treatment as well as when disease progression was suspected. The other exploratory endpoints included the objective response rate (ORR; included the rate of CR and PR), clinical benefit rate (CBR; included the rate of CR, PR and SD ≥ 6 months), and progression-free survival (PFS).
The primary objective of this study was to examine the rate of adverse events in Korean subjects. A sample size of 90 was calculated, assuming the rate of adverse events to be 98.81% with a standard deviation of 2.25% based on the EMBRACE study [4]. After considering a dropout rate of 10%, the final sample size was calculated to be 100.
The primary endpoint was evaluated in the safety set that comprised of patients who had received at least one dose of eribulin. The secondary efficacy endpoint was analyzed using either full analysis set (FAS) or per-protocol set (PPS). The FAS included patients who had at least one primary efficacy evaluation after the baseline, while the PPS included patients without major protocol deviations. The tumor response rate was presented with a 95% confidence interval (CI). The PFS was analyzed using the Kaplan-Meier method and presented with a 95% CI.
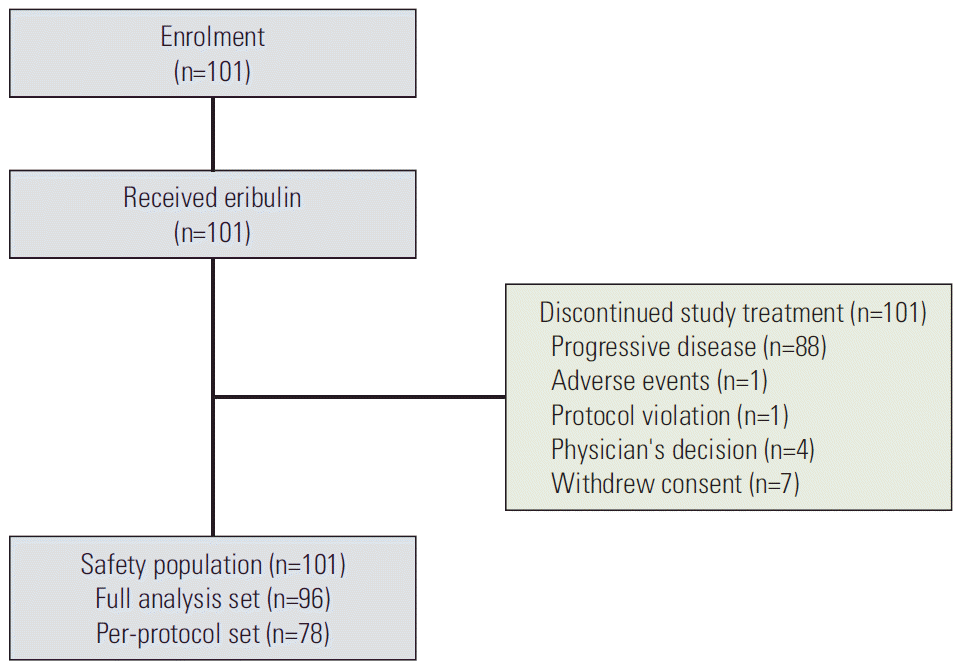
The patients were enrolled between June 2013 and April 2014 from 14 institutes in Korea. Fig. 1 shows the flow of patient enrolment. A total 101 patients received eribulin and were included in the safety set. Thirteen patients discontinued the study prior to progression due to the withdrawal of consent (n=7), protocol violation (n=1), adverse events (n=1), and other reasons (n=4). Five patients were excluded from FAS because there had been no efficacy evaluation after the baseline. Eighteen patients were further excluded from PPS due to inclusion and exclusion criteria deviations (n=10) and other major protocol deviations (n=9).
Table 1 lists the demographic and baseline characteristics of the safety set. The median age was 51 years (range, 25 to 79 years). The median number of previous chemotherapy regimens before study enrolment was four. Other than one patient, who had a contraindication to anthracycline, all patients had received taxane- and anthracycline-included regimens before study enrolment.
In the safety set, a median dose 1.39 mg/m2 (range, 0.71 to 1.44 mg/m2 ) of eribulin was administered each cycle. The patients received a median of three cycles (range, 1 to 31 cycles) of eribulin. The median treatment duration was 2.14 months (range, 0.04 to 23.79 months). During treatment, 40 patients (39.6%) experienced a dose delay and 10 patients (9.9%) experienced a dose reduction due to adverse events.
A total of 982 TEAEs occurred. Among them, 703 events were deemed to be related or caused by the drug investigated according to the investigator assessment. Table 2 summarizes the adverse events with an incidence greater than 10%. Neutropenia of any grade was the most common TEAE and occurred in 92 patients (91.1%) (grade 3, 31.7%; grade 4, 57.4%). Anemia of any grade occurred in 12 patients (11.9%) and thrombocytopenia of any grade occurred in four patients (4.0%) (no case of grade 3 or 4). Among the non-hematologic adverse events, alopecia, decrease in appetite, fatigue/asthenia, and myalgia/arthralgia were frequent. Peripheral neuropathy was encountered in 27 patients (26.7%), including grade 3 in two patients. Pseudomonal sepsis (n=1) was the only grade 4 adverse event observed in this study other than neutropenia. Pseudomonal sepsis occurred on day 8 of the first cycle of eribulin and was resolved with appropriate management.
A total of 27 serious adverse events (SAE) occurred in 20 patients (19.8%), including two each of neutropenia and pericardial effusion. Nervous system disorders were the most commonly reported system organ class with regard to SAE (one event each of consciousness fluctuating, dizziness, headache, neuropathy peripheral, and syncope). Death occurred in one patient due to aspiration pneumonia, which occurred on day 20 of the third cycle of the eribulin treatment. This fatal SAE was considered to be unrelated to eribulin by the investigator.
Table 3 list the response rate evaluated in FAS and PPS. In FAS, DCR was 52.7% (n=49; 95% CI, 42.1 to 63.1), ORR was 17.2% (n=16), and CBR was 21.5% (n=20). In PPS, DCR was 51.3% (n=39; 95% CI, 39.6 to 63.0), ORR was 17.1% (n=13), and CBR was 22.4% (n=17). One patient achieved a CR. Overall, 46.2% (n=43) of patients in FAS and 47.4% (n=36) of patients in PPS had progressive disease. The median PFS was 2.60 months (95% CI, 2.18 to 4.40) in FAS and 2.36 months (95% CI, 2.10 to 4.32) in PPS.
This study examined the safety of eribulin in Korean patients with locally advanced or MBC. Consistent with previous studies, adverse events observed in safety set were generally well tolerated [4,5,7].
A phase I study that administered 1.4 mg/m2 of eribulin on days 1 and 8 of a 21-day cycle identified febrile neutropenia and neutropenia as the dose limiting toxicities [13]. Several phase II and III studies [4,5,7] reported neutropenia as the most common adverse event, as in this study. Regarding the absolute incidence, this study identified a notable difference with the results from previous studies that detected neutropenia as the most frequent adverse event. The incidence of any grade neutropenia was approximately 50% in the EMBRACE study [4] and a study by Kaufman et al. [5], of which the participants comprised mainly of Caucasians at the baseline. On the other hand, it was above 90% in this study and in the study by Aogi et al. [7], which enrolled Asian patients. Similar cases of ethnic differences in the incidence of chemotherapy-induced myelosuppression were also reported in other types of cancer and chemotherapy settings [14-16]. Genetic polymorphisms of the drug metabolizing enzyme and transporter might have contributed to the discordant incidence of adverse events between different ethnicities [17].
Another explanation for the higher incidence of neutropenia is the large number of patients who were heavily pretreated; the median number of prior chemotherapeutic regimens was four (range, 2 to 8). Twenty-three patients (22.8%) were treated with six or more lines of chemotherapeutic regimens (Table 1).
Although 92 patients (91.1%) experienced neutropenia in this study, only one patient (1.0%) experienced febrile neutropenia, which was subsequently resolved with the appropriate management. Of 56 patients (55.5%), granulocyte colony-stimulating factor was required to treat and prevent neutropenia in 55 (54.5%) and 18 (17.8%) patients, respectively.
Unlike neutropenia, there was no significant difference among the incidences of peripheral neuropathy from other studies. The enrolled patients had received taxane- or anthracycline-based chemotherapy before participating in this study. Despite the repetitive chemotherapy treatment history, peripheral neuropathy of grade 3 or more was infrequent (two events of grade 3 and no grade 4) among the patients. The unique mechanism of action of eribulin, which inhibits microtubule polymerization at the plus end while having no effect on depolymerization, may account for the relatively low incidence of peripheral neuropathy [18]. A preclinical study reported that eribulin had a lower tendency to induce new-onset peripheral neuropathy and exacerbate preexisting neuropathy than paclitaxel [19,20]. In a randomized phase II study that compared the incidence and severity of eribulin- or ixabepilone-induced peripheral neuropathy, a lower incidence was recorded in the eribulin arm but the difference was not statistically significant [21].
A relatively lower DCR and shorter PFS in FAS and PPS were observed in this study compared to those in previous studies. The lower response rate and shorter PFS compared to other studies may have resulted from the characteristics of the study population, which had patients with more previous lines of chemotherapy prior to study participation.
The toxicity profile was consistent with previous phase II and phase III studies, and no new adverse events were observed in the Korean patients.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.

2. O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist. 2005; 10 Suppl 3:20–9.
3. Sachdev JC, Jahanzeb M. Use of cytotoxic chemotherapy in metastatic breast cancer: putting taxanes in perspective. Clin Breast Cancer. 2016; 16:73–81.

4. Cortes J, O'Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011; 377:914–23.

5. Kaufman PA, Awada A, Twelves C, Yelle L, Perez EA, Velikova G, et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2015; 33:594–601.

6. Twelves C, Cortes J, Vahdat L, Olivo M, He Y, Kaufman PA, et al. Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies. Breast Cancer Res Treat. 2014; 148:553–61.

7. Aogi K, Iwata H, Masuda N, Mukai H, Yoshida M, Rai Y, et al. A phase II study of eribulin in Japanese patients with heavily pretreated metastatic breast cancer. Ann Oncol. 2012; 23:1441–8.

8. Patel JN. Cancer pharmacogenomics: implications on ethnic diversity and drug response. Pharmacogenet Genomics. 2015; 25:223–30.
9. O’Donnell PH, Dolan ME. Cancer pharmacoethnicity: ethnic differences in susceptibility to the effects of chemotherapy. Clin Cancer Res. 2009; 15:4806–14.

10. Hertz DL, McLeod HL, Irvin WJ Jr. Tamoxifen and CYP2D6: a contradiction of data. Oncologist. 2012; 17:620–30.

11. Swain SM, Im YH, Im SA, Chan V, Miles D, Knott A, et al. Safety profile of pertuzumab with trastuzumab and docetaxel in patients from Asia with human epidermal growth factor receptor 2-positive metastatic breast cancer: results from the phase III trial CLEOPATRA. Oncologist. 2014; 19:693–701.

12. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.

13. Mukohara T, Nagai S, Mukai H, Namiki M, Minami H. Eribulin mesylate in patients with refractory cancers: a Phase I study. Invest New Drugs. 2012; 30:1926–33.

14. Yano R, Konno A, Watanabe K, Tsukamoto H, Kayano Y, Ohnaka H, et al. Pharmacoethnicity of docetaxel-induced severe neutropenia: integrated analysis of published phase II and III trials. Int J Clin Oncol. 2013; 18:96–104.

15. Hasegawa Y, Kawaguchi T, Kubo A, Ando M, Shiraishi J, Isa S, et al. Ethnic difference in hematological toxicity in patients with non-small cell lung cancer treated with chemotherapy: a pooled analysis on Asian versus non-Asian in phase II and III clinical trials. J Thorac Oncol. 2011; 6:1881–8.

16. Han HS, Reis IM, Zhao W, Kuroi K, Toi M, Suzuki E, et al. Racial differences in acute toxicities of neoadjuvant or adjuvant chemotherapy in patients with early-stage breast cancer. Eur J Cancer. 2011; 47:2537–45.

17. Tan SH, Lee SC, Goh BC, Wong J. Pharmacogenetics in breast cancer therapy. Clin Cancer Res. 2008; 14:8027–41.

18. Jordan MA, Kamath K, Manna T, Okouneva T, Miller HP, Davis C, et al. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol Cancer Ther. 2005; 4:1086–95.

19. Wozniak KM, Wu Y, Farah MH, Littlefield BA, Nomoto K, Slusher BS. Neuropathy-inducing effects of eribulin mesylate versus paclitaxel in mice with preexisting neuropathy. Neurotox Res. 2013; 24:338–44.

Table 1.
Demographic and baseline characteristics
| Characteristic | No. (%) (n=101) |
|---|---|
| Age, median (range, yr) | 51 (25-79) |
| ECOG performance status | |
| 0 | 30 (29.7) |
| 1 | 69 (68.3) |
| 2 | 2 (2.0) |
| Diagnosis | |
| Metastatic breast cancer | 99 (98.0) |
| Locally advanced breast cancer | 2 (2.0) |
| HER2a) | |
| Positive | 21 (20.8) |
| Negative | 80 (79.2) |
| ER and PR status | |
| ER and/or PR positive | 62 (61.4) |
| ER and PR negative | 39 (38.6) |
| ER, PR, HER2 negative | 30 (29.7) |
| Most common metastatic sites | |
| Lymph node | 78 (77.2) |
| Lung | 65 (64.4) |
| Bone | 57 (56.4) |
| Liver | 54 (53.5) |
| Brain | 12 (11.9) |
| No. of previous chemotherapy regimens | |
| 2 | 1 (1.0) |
| 3 | 20 (19.8) |
| 4 | 36 (35.6) |
| 5 | 21 (20.8) |
| ≥ 6 | 23 (22.8) |
| Median (range) | 4 (2-8) |
| No. of previous chemotherapy regimens in advanced setting | |
| 2 | 28 (27.7) |
| 3 | 30 (29.7) |
| 4 | 24 (23.8) |
| 5 | 18 (17.8) |
| 6 | 1 (1.0) |
| Median | 3 (2-8) |
| Previous chemotherapyb) | |
| Taxanes | 101 (100) |
| Anthracyclines | 100 (99.0) |
| Previous surgery | 94 (93.1) |
| Previous radiotherapy | 82 (81.2) |
Table 2.
Adverse events with an incidence higher than 10%
|
No. (%) (n=101) |
|||
|---|---|---|---|
| All grades | Grade 3 | Grade 4 | |
| Hematological | |||
| Neutropenia | 92 (91.1) | 32 (31.7) | 58 (57.4) |
| Anemia | 12 (11.9) | 3 (3.0) | 0 |
| Leukopenia | 11 (10.9) | 6 (5.9) | 0 |
| Nonhematological | |||
| Alopecia | 46 (45.5) | NA | |
| Decrease of appetite | 42 (41.6) | 1 (1.0) | 0 |
| Fatigue/Asthenia | 33 (32.7) | 4 (4.0) | 0 |
| Peripheral neuropathya) | 27 (26.7) | 2 (2.0) | 0 |
| Myalgia/Arthralgia | 26 (25.7) | 1 (1.0) | 0 |
| Nausea | 25 (24.8) | 0 | 0 |
| Cough | 19 (18.8) | 1 (1.0) | 0 |
| Pyrexia | 18 (17.8) | 0 | 0 |
| Headache | 13 (12.9) | 1 (1.0) | 0 |
| Vomiting | 11 (10.9) | 2 (2.0) | 0 |
Table 3.
Disease control rate
| Best overall tumor response |
Investigator review |
|
|---|---|---|
| FAS | PPS | |
| Tumor response | ||
| Complete response | 1 (1.1) | 1 (1.3) |
| Partial response | 15 (16.1) | 12 (15.8) |
| Stable disease | 33 (35.5) | 26 (34.2) |
| Progressive disease | 43 (46.2) | 36 (47.4) |
| Not evaluable | 1 (1.1) | 1 (1.3) |
| Disease control ratea) | 49 (52.7) | 39 (51.3) |
| 95% Confidence interval | 42.06-63.14 | 39.57-62.96 |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download