1. Shin HR. Global activity of cancer registries and cancer control and cancer incidence statistics in Korea. J Prev Med Public Health. 2008; 41:84–91.

2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.

3. Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015; 47:127–41.

4. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. All cancers (excluding nonmelanoma skin cancer) estimated incidence, mortality and prevalence worldwide in 2012 [Internet]. Geneva: World Health Organization;2016. [cited 2016 Mar 1]. Available from:
http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx#.
5. Park SK, Sakoda LC, Kang D, Chokkalingam AP, Lee E, Shin HR, et al. Rising prostate cancer rates in South Korea. Prostate. 2006; 66:1285–91.

6. Cheon J, Kim CS, Lee ES, Hong SJ, Cho YH, Shin EC, et al. Survey of incidence of urological cancer in South Korea: a 15-year summary. Int J Urol. 2002; 9:445–54.

7. Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al. International classification of diseases for oncology. 3rd ed. Geneva: World Health Organization;2000.
8. World Health Organization. International statistical classification of diseases and related health problems. 10th rev. Geneva: World Health Organization;1994.
9. Shin HR, Won YJ, Jung KW, Kong HJ, Yim SH, Lee JK, et al. Nationwide cancer incidence in Korea, 1999~2001; first result using the national cancer incidence database. Cancer Res Treat. 2005; 37:325–31.

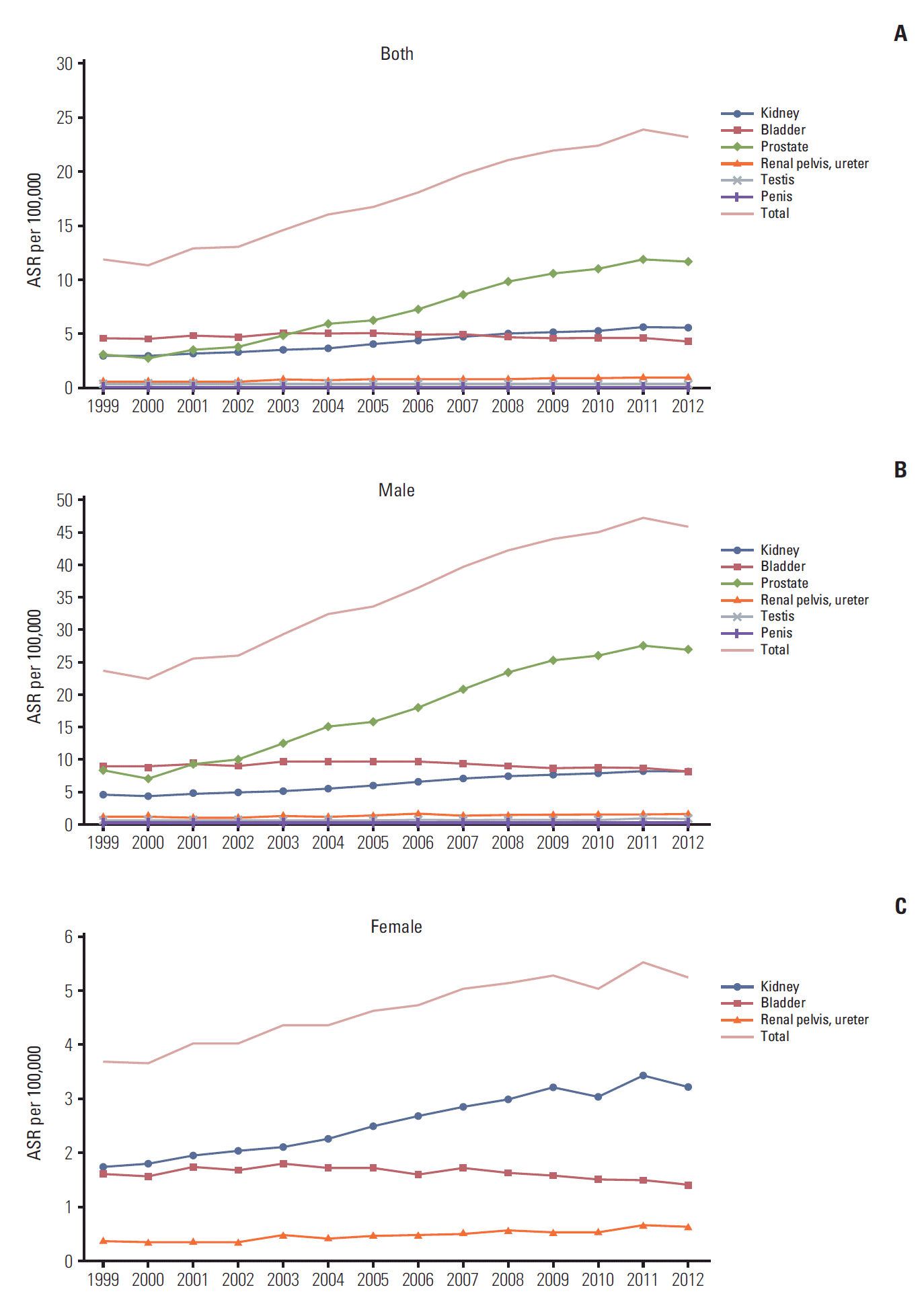
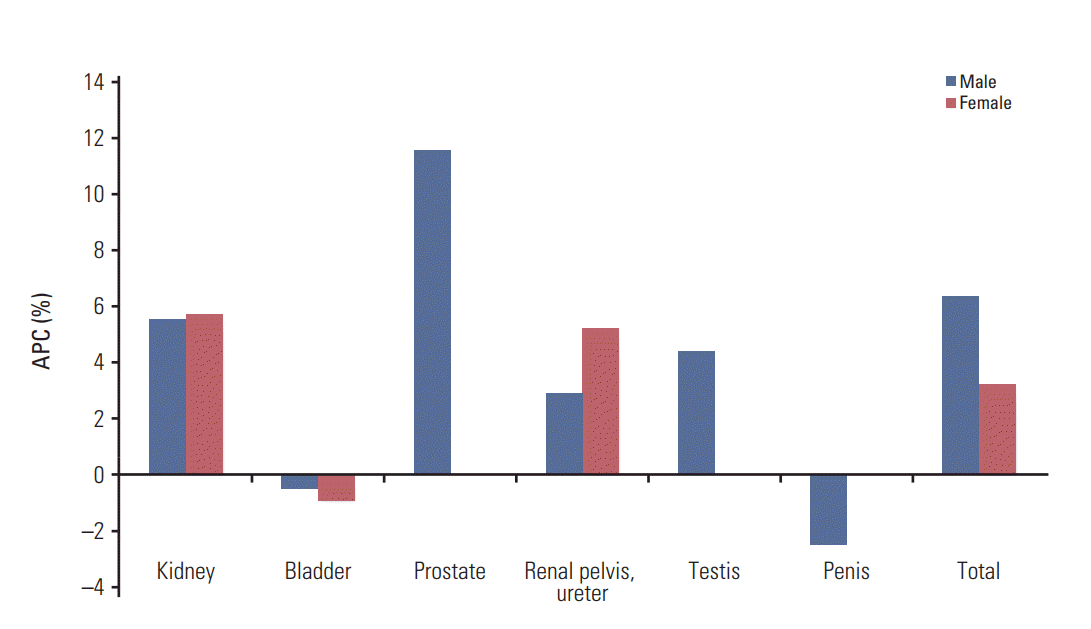
10. Song W, Jeon HG. Incidence of kidney, bladder, and prostate cancers in Korea: an update. Korean J Urol. 2015; 56:422–8.

11. Huncharek M, Haddock KS, Reid R, Kupelnick B. Smoking as a risk factor for prostate cancer: a meta-analysis of 24 prospective cohort studies. Am J Public Health. 2010; 100:693–701.

12. Giovannucci E, Rimm EB, Colditz GA, Stampfer MJ, Ascherio A, Chute CG, et al. A prospective study of dietary fat and risk of prostate cancer. J Natl Cancer Inst. 1993; 85:1571–9.

13. Katanoda K, Hori M, Matsuda T, Shibata A, Nishino Y, Hattori M, et al. An updated report on the trends in cancer incidence and mortality in Japan, 1958-2013. Jpn J Clin Oncol. 2015; 45:390–401.

14. Andriole GL, Crawford ED, Grubb RL 3rd, Buys SS, Chia D, Church TR, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012; 104:125–32.
15. Choi SK, Song C, Shim M, Min GE, Park J, Jeong IG, et al. Prevalence of high-grade or insignificant prostate cancer in Korean men with prostate-specific antigen levels of 3.0-4.0 ng/mL. Urology. 2015; 85:610–5.
16. Jeong IG, Dajani D, Verghese M, Hwang J, Cho YM, Hong JH, et al. Differences in the aggressiveness of prostate cancer among Korean, Caucasian, and African American men: A retrospective cohort study of radical prostatectomy. Urol Oncol. 2016; 34:3.

17. Morrison AS, Buring JE, Verhoek WG, Aoki K, Leck I, Ohno Y, et al. An international study of smoking and bladder cancer. J Urol. 1984; 131:650–4.

18. Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010; 7:245–57.

19. King SC, Pollack LA, Li J, King JB, Master VA. Continued increase in incidence of renal cell carcinoma, especially in young patients and high grade disease: United States 2001 to 2010. J Urol. 2014; 191:1665–70.

20. Adams KF, Leitzmann MF, Albanes D, Kipnis V, Moore SC, Schatzkin A, et al. Body size and renal cell cancer incidence in a large US cohort study. Am J Epidemiol. 2008; 168:268–77.

21. Park HS, Park CY, Oh SW, Yoo HJ. Prevalence of obesity and metabolic syndrome in Korean adults. Obes Rev. 2008; 9:104–7.

22. Pike MC, Chilvers CE, Bobrow LG. Classification of testicular cancer in incidence and mortality statistics. Br J Cancer. 1987; 56:83–5.

23. Bleeker MC, Heideman DA, Snijders PJ, Horenblas S, Dillner J, Meijer CJ. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009; 27:141–50.

24. Larke NL, Thomas SL, dos Santos Silva I, Weiss HA. Male circumcision and penile cancer: a systematic review and meta-analysis. Cancer Causes Control. 2011; 22:1097–110.

25. Minhas S, Manseck A, Watya S, Hegarty PK. Penile cancer--prevention and premalignant conditions. Urology. 2010; 76(2 Suppl 1):S24–35.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download