1. Di Croce L, Raker VA, Corsaro M, Fazi F, Fanelli M, Faretta M, et al. Methyltransferase recruitment and DNA hypermethylation of target promoters by an oncogenic transcription factor. Science. 2002; 295:1079–82.

2. Nobori T, Miura K, Wu DJ, Lois A, Takabayashi K, Carson DA. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994; 368:753–6.

3. Teofili L, Martini M, Di Mario A, Rutella S, Urbano R, Luongo M, et al. Expression of p15(ink4b) gene during megakaryocytic differentiation of normal and myelodysplastic hematopoietic progenitors. Blood. 2001; 98:495–7.

4. Bergh G, Ehinger M, Olsson I, Jacobsen SE, Gullberg U. Involvement of the retinoblastoma protein in monocytic and neutrophilic lineage commitment of human bone marrow progenitor cells. Blood. 1999; 94:1971–8.

5. Haviernik P, Schmidt M, Hu X, Wolff L. Consistent inactivation of p19(Arf) but not p15(Ink4b) in murine myeloid cells transformed in vivo by deregulated c-Myc. Oncogene. 2003; 22:1600–10.

6. Teofili L, Morosetti R, Martini M, Urbano R, Putzulu R, Rutella S, et al. Expression of cyclin-dependent kinase inhibitor p15(INK4B) during normal and leukemic myeloid differentiation. Exp Hematol. 2000; 28:519–26.

7. Aggerholm A, Guldberg P, Hokland M, Hokland P. Extensive intra- and interindividual heterogeneity of p15INK4B methylation in acute myeloid leukemia. Cancer Res. 1999; 59:436–41.
8. Rush LJ, Plass C. Alterations of DNA methylation in hematologic malignancies. Cancer Lett. 2002; 185:1–12.

9. Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposed revised criteria for the classification of acute myeloid leukemia: a report of the French-American-British Cooperative Group. Ann Intern Med. 1985; 103:620–5.
10. Chim CS, Wong AS, Kwong YL. Epigenetic inactivation of INK4/CDK/RB cell cycle pathway in acute leukemias. Ann Hematol. 2003; 82:738–42.

11. Drexler HG. Review of alterations of the cyclin-dependent kinase inhibitor INK4 family genes p15, p16, p18 and p19 in human leukemia-lymphoma cells. Leukemia. 1998; 12:845–59.

12. Herman JG, Jen J, Merlo A, Baylin SB. Hypermethylation-associated inactivation indicates a tumor suppressor role for p15INK4B. Cancer Res. 1996; 56:722–7.
13. Issa JP, Baylin SB, Herman JG. DNA methylation changes in hematologic malignancies: biologic and clinical implications. Leukemia. 1997; 11 Suppl 1:S7–11.
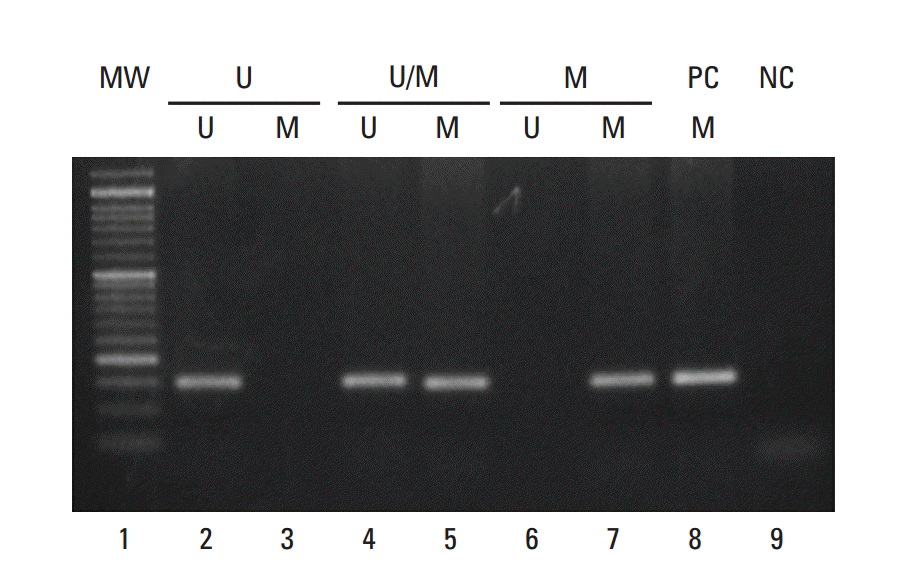
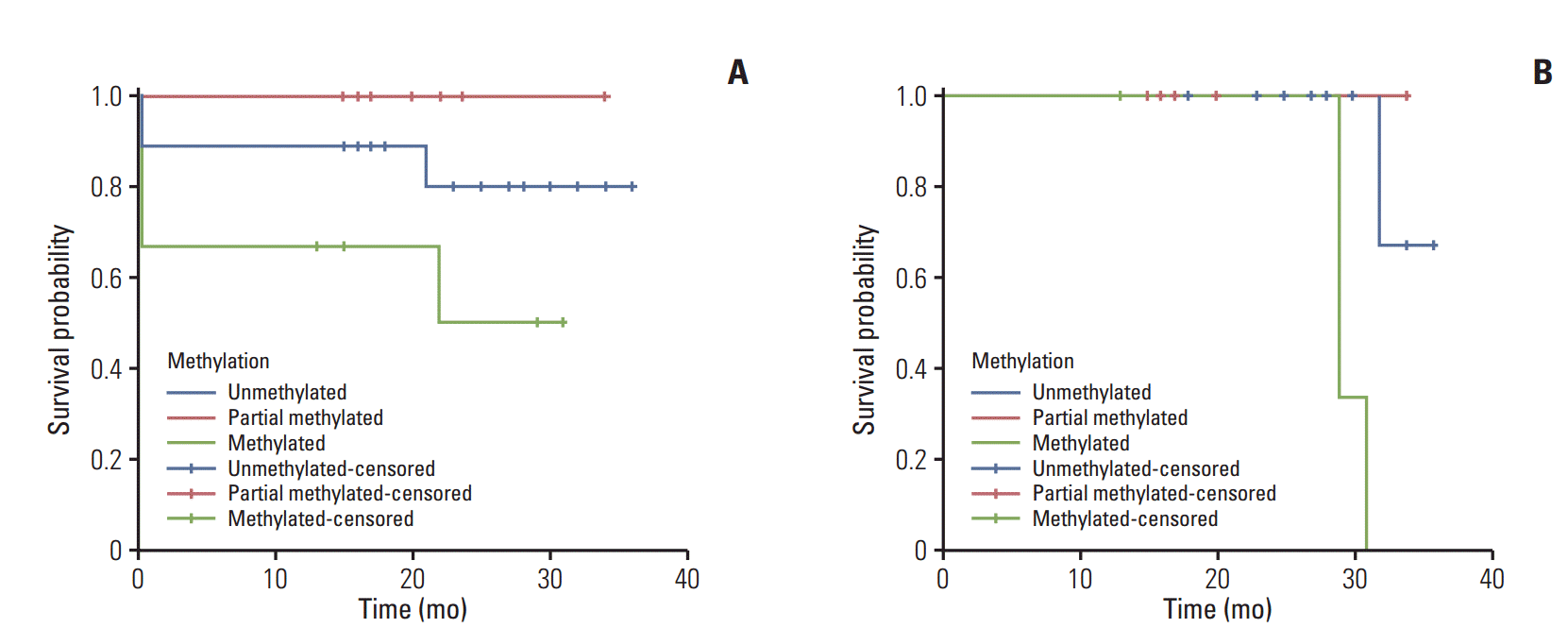
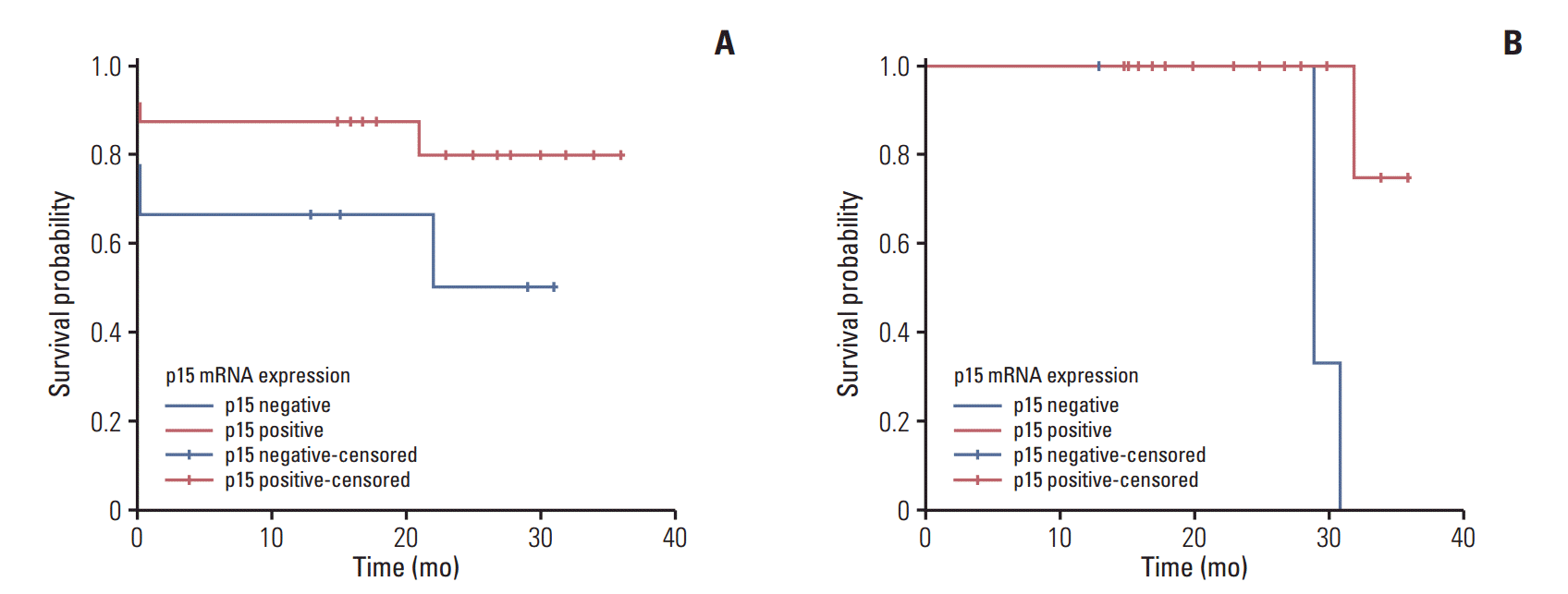
14. Teofili L, Martini M, Luongo M, Diverio D, Capelli G, Breccia M, et al. Hypermethylation of GpG islands in the promoter region of p15(INK4b) in acute promyelocytic leukemia represses p15(INK4b) expression and correlates with poor prognosis. Leukemia. 2003; 17:919–24.

15. Chim CS, Wong SY, Kwong YL. Aberrant gene promoter methylation in acute promyelocytic leukaemia: profile and prognostic significance. Br J Haematol. 2003; 122:571–8.

16. Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002; 100:2292–302.

17. Trost D, Hildebrandt B, Beier M, Muller N, Germing U, Royer-Pokora B. Molecular cytogenetic profiling of complex karyotypes in primary myelodysplastic syndromes and acute myeloid leukemia. Cancer Genet Cytogenet. 2006; 165:51–63.

18. Asou N, Adachi K, Tamura J, Kanamaru A, Kageyama S, Hiraoka A, et al. Analysis of prognostic factors in newly diagnosed acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy. Japan Adult Leukemia Study Group. J Clin Oncol. 1998; 16:78–85.

19. Ferrara F, Morabito F, Martino B, Specchia G, Liso V, Nobile F, et al. CD56 expression is an indicator of poor clinical outcome in patients with acute promyelocytic leukemia treated with simultaneous all-trans-retinoic acid and chemotherapy. J Clin Oncol. 2000; 18:1295–300.

20. Preisler HD, Li B, Chen H, Fisher L, Nayini J, Raza A, et al. P15INK4B gene methylation and expression in normal, myelodysplastic, and acute myelogenous leukemia cells and in the marrow cells of cured lymphoma patients. Leukemia. 2001; 15:1589–95.

21. Shimamoto T, Ohyashiki JH, Ohyashiki K. Methylation of p15(INK4b) and E-cadherin genes is independently correlated with poor prognosis in acute myeloid leukemia. Leuk Res. 2005; 29:653–9.

22. Deneberg S, Grovdal M, Karimi M, Jansson M, Nahi H, Corbacioglu A, et al. Gene-specific and global methylation patterns predict outcome in patients with acute myeloid leukemia. Leukemia. 2010; 24:932–41.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download