Abstract
Purpose
The purpose of this study was to study the clinical outcome for patients with metastases of the adrenal gland treated with stereotactic body radiation therapy.
Materials and Methods
Forty-six patients were studied retrospectively. The dose prescription was 40 Gy in four fractions. Dosimetric analysis was performed using the dose volume histograms while clinical outcome was assessed using actuarial analysis with determination of the overall survival (OS) and local control (LC) rates.
Results
The planning objectives were met for all patients. With a median follow-up period of 7.6 months, at the last follow-up 42 patients (91.3%) were alive and four had died because of distant progression. The actuarial mean OS was 28.5±1.6 months, the median was not reached. One-year and 2-year OS were 87.6±6.1%. None of the risk factors was significant in univariate analysis. Actuarial mean LC was 14.6±1.8 months (95% confidence interval [CI], 11.0 to 18.2) and median LC was 14.5±2.0 months (95% CI, 10.5 to 18.5). One-year and 2-year LC were 65.5±11.9% and 40.7±15.8%, respectively. A mild profile of toxicity was observed in the cohort of patients. Forty patients (86.9%) showed no complication (grade 0); two patients reported asthenia, six patients (13.1%) reported either pain, nausea, or vomiting. Of these six patients, five patients (10.9%) were scored as grade 1 toxicity while one patient (2.2%) was scored as grade 2.
Adrenal glands are a common site for metastatic spread, with autopsy studies documenting their presence in 27%-38% of all fatal malignancies [1]. Various primary tumors can metastasize to the adrenal glands; approximately 50% of melanomas, 30%-40% of breast and lung cancers, and 10%-20% of renal and gastrointestinal tumors involve this site during their natural history [2]. Adrenal gland metastases rank fourth, worldwide, after lung, liver, and bone metastases [3]. Although clinically occult in most cases, their diagnosis is becoming more common with the technological improvement of diagnostic tools and the strict follow-up schedules for such cancer patients [4]. Therefore, the diagnosis of adrenal metastases is often made at a very early stage, with most patients still in an oligometastatic state.
The oligometastatic state [5] is a disease state intermediate between loco-regionally confined and widely metastatic cancer. Increasing evidence from the literature suggests that selected patients with oligometastases may remain in a disease-free status for more than a decade if treated with an aggressive combined-modality therapy, including both systemic and local approach. Due to the absence of prospective trials and the paucity of retrospective series, it is still unclear whether this also applies to adrenal metastases. The best evidence suggesting a survival advantage from local treatment of adrenal metastases can be derived from retrospective case-control studies comparing adrenalectomy plus systemic therapy versus systemic therapy alone. Each study found significantly prolonged median overall survival (OS) in the adrenalectomy patient arm; however, patient selection likely played a pivotal role [6-8].
Adrenalectomy is currently the most frequent treatment approach [9,10]. In a meta-analysis of 114 patients with non-small cell lung cancer undergoing resection of adrenal metastases, 5-year OS was 25%-26%, confirming that there can be long-term survivors in a selected group of patients [11]. Although the surgical approach can be considered as a gold standard, it is not free from severe complications, including infections (3.8%-12%), myocardial infarction (2%), ileus (7%), and even death (2%) [11], and the contraindications to a surgical approach, including age or other comorbidities, remain a significant limitation.
Several alternative approaches have been explored in recent years, including radiofrequency ablation (RFA) [12] or trans-arterial chemoembolization [13]. However, longer median survival and OS times were demonstrated with resection of clinically isolated adrenal metastases when compared with non-surgical therapy, including RFA, external beam radiotherapy, arterial embolization, radio-embolization, bland embolization, chemical ablation, and cryo-ablation [14].
The role of radiotherapy (RT) has historically been limited to a palliative intent [15,16]. However, recent technological improvement with the introduction of stereotactic body radiation therapy (SBRT) increased the competitiveness of this local ablative treatment. SBRT enables the delivery of very high doses of radiation to tumor cells in a few fractions, while sparing the surrounding healthy tissues. This feature is particularly important in an anatomical area like the abdomen, where the normal tissue dose-volume constraints limited the efficacy of RT for years. Dose escalation with SBRT is possible and results in outcome data comparable to that achieved with surgery [17].
The aim of the current study is to report on the treatment outcome of patients treated with SBRT with volumetric modulated arc therapy (VMAT) for adrenal gland metastases, with special focus on local control, toxicity, and survival.
Between 2011 and 2015, 46 patients with adrenal gland metastases were treated with SBRT in our institute. Data collection and the analysis for an observational retrospective study were approved by the institutional review board based on an analysis of charts. All patients were treated in accordance with the Helsinki declaration. The inclusion criteria for the SBRT treatment were as follows: age greater than 18 years, World Health Organization performance status ≤ 2, histologically-proven primary cancer disease, M1 stage with primary cancer radically treated with complete or stable response, a maximum of five metastases, a lesion diameter < 5 cm, no previous radiation treatment or surgical intervention in the region. All patients signed an informed consent at registration.
Free breathing 4D computed tomography (4DCT) scans with 3-mm slice thickness were acquired for treatment planning in supine position, with the patient’s arms above the head. Thermoplastic chest masks with abdominal compression (made with the insertion of styrofoam blocks under the mask in correspondence with the diaphragm) were constructed to improve the patient immobilization and reduce the motion of internal organs. The clinical target volume (CTV) included the metastases’ mass as identified on the computed tomography (CT) images. Set-up margins were added with an isotropic expansion of 5 mm from the envelope of the CTV volumes reconstructed in all phases of the 4DCT. The new volume was labelled as the planning target volume (PTV). The organs at risk (OAR) defined included the stomach and the duodenum, the small bowel, the liver, the spinal cord, and the kidneys.
The dose prescription was 40.0 Gy in four daily fractions of 10 Gy (mean dose to CTV) for all patients. This prescription is a dose escalation from the earlier regimen of 45 Gy in six fractions of 7.5 Gy reported in the feasibility study [18]. The planning objectives for the CTV were V98% > 98% and the minimization of the near-to-maximum dose (D1%). For PTV the coverage requirement was relaxed to V95% > 90% and possibly further reduced for individual challenging cases. For the OARs, beside a general request for minimization of the mean and the near-to-maximum doses, the following explicit objectives were considered: (1) liver: total liver volume, V15Gy > 700 cm3; (2) spinal cord: D1% < 18 Gy; (3) kidneys (ipsilateral): V15Gy < 35%; and (4) duodenum: D1% < 36 Gy.
SBRT treatments were performed using the RapidArc ver. of VMAT on a TrueBeam linear accelerator (Varian Medical Systems, Palo Alto, CA) selecting the flattening filter free photon beams of 6 or 10 MV with a dose rate of 1,400 or 2,400 MU/min respectively to minimize the treatment time. The treatment plans were optimized using the Eclipse system ver.11 (Varian Medical Systems). The daily patient set-up was controlled using 3D cone beam CT images compared against the reconstructed image from the 4DCT planning scans. A free-breathing delivery was selected for all patients. Further details regarding the planning procedure and the dosimetric features of the treatment can be found in the feasibility investigation report published earlier [18].
All treatment plans were appraised by analysis of the dose volume histograms. The clinical outcome was evaluated during the periodic follow-up visits; CT scans were acquired at 1 month after treatment and then every 3 months. For a group of 14 patients (30%), a positron emission tomography scan was performed using 18F-fluorodeoxyglucose 6 months after the end of treatment. The radiological response was defined according to the Response Evaluation Criteria in Solid Tumors criteria and reported at the time of the maximal response. Toxicity was recorded using the Common Terminology Criteria for Adverse Events ver. 4.0. Descriptive statistics was used for characterization of the cohort data. The local control (LC) and OS rates were computed using the Kaplan-Meier analysis and univariate analysis performed using log-rank tests. The variables tested were age, sex, performance status, laterality, primary tumour histology, solitary metastasis versus oligometastatic status. SPSS software ver. 22 (IBM Corp., Armonk, NY) was used for the tests.
The patient’s characteristics are summarized in Table 1. The median follow-up period from SBRT was 7.6 months with a mean of 11.3 months.
Fig. 1 shows the dose distribution (in color-wash) for one typical patient from the cohort in the axial, sagittal, and coronal planes. The analysis of the dose volume histograms (DVH) from the treatment plans reporting the dosimetric parameters subject to planning objectives for the various structures is summarized in Table 2. Fig. 2 shows the average DVH for the targets and the OAR (solid line); the interpatient variability is represented at 1 standard deviation by the dashed lines. Each line in the table reports one dose-volume constraints used for the optimization of the treatment plans while each column reports the means observed for the various OAR or target volumes for which the constraint was applied. For liver and kidneys, the data are reported only for the “ipsilateral” organ with respect to the treated lesion. The planning objectives were met for all patients.
At the last follow-up, 42 patients (91.3%) were alive, four patients (8.7%) died because of the disease. The crude local response resulted in 15 patients (32.6%) with complete response, 21 patients (45.6%) with partial response or stable disease, and 10 patients (21.7%) with local progression of the disease. Considering only the subgroup of patients with primary lung tumor, an overall benefit rate of 86.6% was observed. The four patients (13.3%) experiencing progression of the disease during the follow-up period were affected by lung adenocarcinoma (n=2) and small cell lung cancer (n=2). While the first two were still alive at the time of the analysis, the latter died because of the disease. Progression of metastasis after treatment was also observed in one patient affected by neuroendocrine carcinoma of the prostate and urothelial carcinoma of the bladder.
Accounting for the group of 20 patients with solitary single metastasis in the adrenal gland without other sites of disease, local progression of the disease was observed in five patients with a mean period of 6.7 months. On the contrary, new sites of distant metastasis appeared in 11 cases (55%) with a mean period of 5.2 months. Seven out of 20 patients (35%) were free from disease at the time of the analysis, two (10%) with only disease in the adrenal gland. Twenty-six patients (56.5%) had more than one metastasis at the time of treatment; all patients benefitted from SBRT but two showed local progression after 9.1 and 11.3 months.
Distant progression was observed in 27 patients (58.7%) with new metastases identified in other organs after SBRT treatment for the adrenal localization. The sites of distant progression included the lungs in 13 patients (48%), the liver in five patients (19%), the bones in four patients (15%) plus other localizations (e.g., brain, pelvic nodes, and bladder) with lower incidence. All four patients who died presented distant progression but only one also presented with local progression.
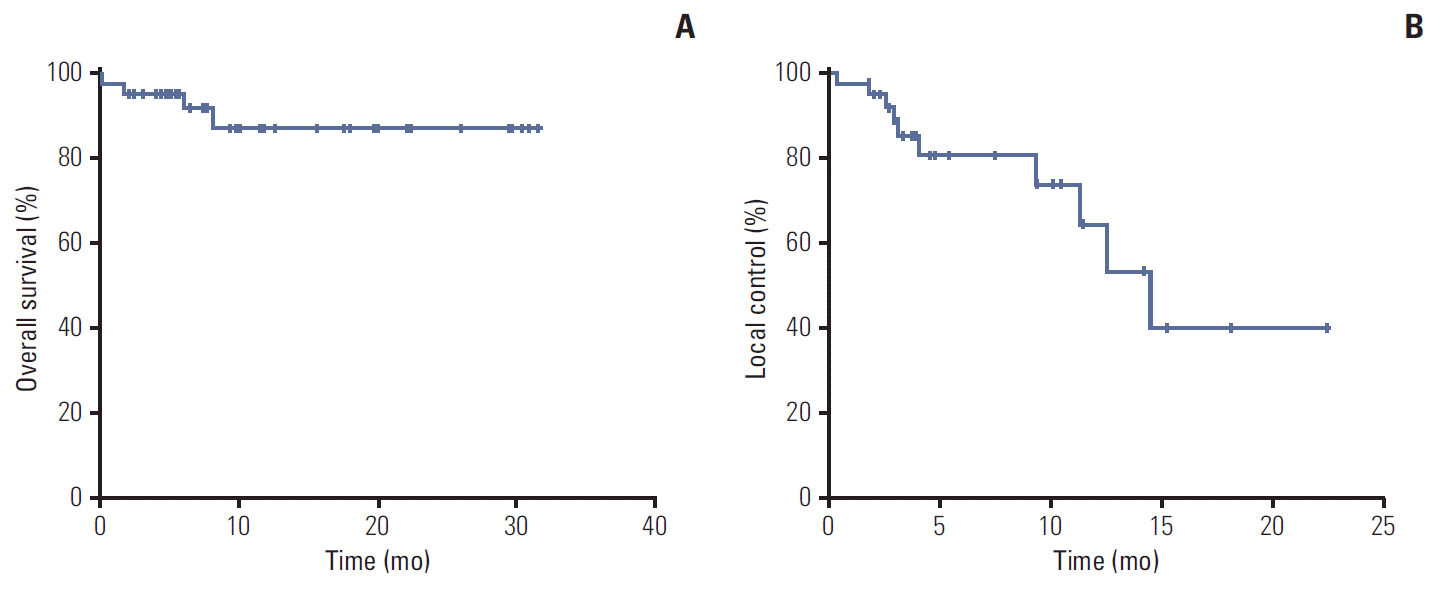
The actuarial mean OS was 28.5±1.6 months (95% confidence interval [CI], 25.4 to 31.6), median was not reached. The 1-year and 2-year OS were 87.6±6.1%. None of the risk factors showed a significant result in the univariate analysis. The actuarial mean LC was 14.6±1.8 months (95% CI, 11.0 to 18.2) and the median LC was 14.5±2.0 months (95% CI, 10.5 to 18.5). The 1-year and 2-year LC were 65.5±11.9% and 40.7±15.8%, respectively. In this case, no risk factors were distinguished among the subgroups of patients. The graphs for the actuarial OS and LC are shown in Fig. 3.
A mild profile of toxicity was observed in the cohort of patients. Forty patients (86.9%) showed no complication (grade 0). Two patients reported asthenia, six patients (13.1%) reported either pain, nausea, or vomiting. Of these six patients, five (10.9%) were scored as grade 1 toxicity while one (2.2%) was scored as grade 2. All toxicity symptoms recovered with simple medication.
Although metastases to the adrenal glands are common, optimal management is still uncertain as clinical evidence is limited. A systematic review was recently published on the role of surgical and ablative therapies for oligometastatic patients with adrenal metastases [19]. The analysis demonstrated 2-year OS rates in favor of surgery compared to SBRT (44% vs. 19%); however, the authors stressed that this difference in survival might be limited by several factors. First, the patient’s selection was biased by performance status, co-morbidities, and uncontrolled extra-adrenal disease. In fact, operated patients are commonly characterized by better condition and fewer comorbidities, while extra-adrenal disease was more common in the SBRT group compared to the surgery group (52% vs. 25%). In addition, the most common primary tumor in the SBRT series (68%) was in the lung and metastatic lung disease has a worse prognosis than other malignancies. So far, RT has been regarded as a palliative care or a “last chance care.”
Another weak point against radiotherapy is the heterogeneity of the prescription doses and fractionation regimens. Indeed, even if high doses can be administered in a few fractions sparing the most relevant OARs with SBRT, it is still unclear which one is the best schedule. To the best of our knowledge, the current report is the only one with consistent dose and fractionation. For all patients a total dose of 40 Gy in four fractions of 10 Gy each was delivered, representing a biological effective dose (BED10) of 80 Gy. A higher BED was administered only by Casamassima et al. [20]; however, the schedules used by this group were quite heterogeneous, because single fraction and multi-fraction stereotactic radiotherapy were included. The dose range varied from 21 to 54 Gy, reaching a median BED10 of 137.3 Gy. The authors showed an actuarial LC rate of 90% at 2 years but an OS of 14.5%.
Chawla et al. [21] treated a smaller sample; BED10 varied from 22.4 Gy (16 Gy in 4 fractions) to 75 Gy (50 Gy in 10 fractions) and the 1-year OS and LC were 44% and 55%, respectively.
Considering the results of these two studies with an acceptable number of cases, and comparing them with the current data, it is clear that BED10 influences the LC. In fact [21,22], a BED10 greater than 100 Gy is an important parameter to obtaining good control of disease; a BED10 of 80 Gy results in an interesting but still unsatisfactory LC. The OS reported in our sample was the highest among the studies in the literature (87.6% at 1 and 2 years); however, this outcome can be influenced by the percentage of primaries included in the analysis. A summary of the most relevant studies reporting on SBRT treatment of the adrenal gland, including the current one, is shown in Table 3.
In terms of toxicity, patients tolerated the treatment well without interruption. The total dose was administered in four consecutive days without interruptions. The compliance with treatment was optimal and the most common side effects were mild asthenia, nausea, and vomiting (grade 1 or 2); no grade 3 or 4 toxicities were observed. Several studies on surgical approaches were also published. In a recent study by Romero Arenas et al. [26] analyzing the benefit of adrenalectomy in the management of adrenal metastases, the median OS was 2.46 years and the 1- and 3-year survival were 70% and 40%, respectively. Complications were not infrequent in this group, both intraoperatively (e.g., bleeding, pneumothorax, and superior vena cava syndrome) and postoperatively (e.g., nerve injury, hematoma, and prolonged ileus). Even if the selection of the patients as candidates for surgery is not well established, this approach can preferably be reserved to smaller organ-confined lesions with no apparent involvement of the adrenal capsule or vascular pedicle.
Modern techniques of radiotherapy, such as VMAT, enable highly precise and rapid treatments respecting the dose constraints for all organs close to the affected gland. Although retrospective, our analysis on a homogeneous group of patients confirmed the promising local control rates achievable by means of SBRT with VMAT for adrenal metastases. The dose escalation applied in this study, compared with the feasibility phase, enabled management of the entire treatment within one single week with good compliance from the patients and minimum distress induced by the small number of fractions. No endocrinology assessment was performed so far, this evaluation will be included in a prospective trial, which could also help to confirm the optimal dose in a larger number of patients.
References
1. Lam KY, Lo CY. Metastatic tumours of the adrenal glands: a 30-year experience in a teaching hospital. Clin Endocrinol (Oxf). 2002; 56:95–101.

2. Wansaicheong G, Goh J. Adrenal metastases [Internet]. New York: Medsacape;2016. [cited 2016 Mar 2]. Available from: http://www.emedicine.com/radio/TOPIC17.
3. Oshiro Y, Takeda Y, Hirano S, Ito H, Aruga T. Role of radiotherapy for local control of asymptomatic adrenal metastasis from lung cancer. Am J Clin Oncol. 2011; 34:249–53.

4. Kumar R, Xiu Y, Yu JQ, Takalkar A, El-Haddad G, Potenta S, et al. 18F-FDG PET in evaluation of adrenal lesions in patients with lung cancer. J Nucl Med. 2004; 45:2058–62.
5. Hellman S. Karnofsky Memorial Lecture: natural history of small breast cancers. J Clin Oncol. 1994; 12:2229–34.

6. Luketich JD, Burt ME. Does resection of adrenal metastases from non-small cell lung cancer improve survival? Ann Thorac Surg. 1996; 62:1614–6.

7. Mittendorf EA, Lim SJ, Schacherer CW, Lucci A, Cormier JN, Mansfield PF, et al. Melanoma adrenal metastasis: natural history and surgical management. Am J Surg. 2008; 195:363–8.

8. Muth A, Persson F, Jansson S, Johanson V, Ahlman H, Wangberg B. Prognostic factors for survival after surgery for adrenal metastasis. Eur J Surg Oncol. 2010; 36:699–704.

9. Kim SH, Brennan MF, Russo P, Burt ME, Coit DG. The role of surgery in the treatment of clinically isolated adrenal metastasis. Cancer. 1998; 82:389–94.

10. Uberoi J, Munver R. Surgical management of metastases to the adrenal gland: open, laparoscopic, and ablative approaches. Curr Urol Rep. 2009; 10:67–72.

11. Tanvetyanon T, Robinson LA, Schell MJ, Strong VE, Kapoor R, Coit DG, et al. Outcomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in non-small-cell lung cancer: a systematic review and pooled analysis. J Clin Oncol. 2008; 26:1142–7.

12. Wood BJ, Abraham J, Hvizda JL, Alexander HR, Fojo T. Radiofrequency ablation of adrenal tumors and adrenocortical carcinoma metastases. Cancer. 2003; 97:554–60.

13. Hsieh MH, Lin ZY, Huang CJ, Shih MC, Chuang WL. Management of bilateral adrenal metastases from hepatocellular carcinoma: a case report. Kaohsiung J Med Sci. 2005; 21:371–6.

15. Soffen EM, Solin LJ, Rubenstein JH, Hanks GE. Palliative radiotherapy for symptomatic adrenal metastases. Cancer. 1990; 65:1318–20.

16. Short S, Chaturvedi A, Leslie MD. Palliation of symptomatic adrenal gland metastases by radiotherapy. Clin Oncol (R Coll Radiol). 1996; 8:387–9.

17. Alongi F, Arcangeli S, Filippi AR, Ricardi U, Scorsetti M. Review and uses of stereotactic body radiation therapy for oligometastases. Oncologist. 2012; 17:1100–7.

18. Scorsetti M, Mancosu P, Navarria P, Tozzi A, Castiglioni S, Clerici E, et al. Stereotactic body radiation therapy (SBRT) for adrenal metastases: a feasibility study of advanced techniques with modulated photons and protons. Strahlenther Onkol. 2011; 187:238–44.
19. Gunjur A, Duong C, Ball D, Siva S. Surgical and ablative therapies for the management of adrenal 'oligometastases': a systematic review. Cancer Treat Rev. 2014; 40:838–46.
20. Casamassima F, Livi L, Masciullo S, Menichelli C, Masi L, Meattini I, et al. Stereotactic radiotherapy for adrenal gland metastases: university of Florence experience. Int J Radiat Oncol Biol Phys. 2012; 82:919–23.

21. Chawla S, Chen Y, Katz AW, Muhs AG, Philip A, Okunieff P, et al. Stereotactic body radiotherapy for treatment of adrenal metastases. Int J Radiat Oncol Biol Phys. 2009; 75:71–5.

22. Katoh N, Onimaru R, Sakuhara Y, Abo D, Shimizu S, Taguchi H, et al. Real-time tumor-tracking radiotherapy for adrenal tumors. Radiother Oncol. 2008; 87:418–24.

23. Torok J, Wegner RE, Burton SA, Heron DE. Stereotactic body radiation therapy for adrenal metastases: a retrospective review of a noninvasive therapeutic strategy. Future Oncol. 2011; 7:145–51.

24. Holy R, Piroth M, Pinkawa M, Eble MJ. Stereotactic body radiation therapy (SBRT) for treatment of adrenal gland metastases from non-small cell lung cancer. Strahlenther Onkol. 2011; 187:245–51.

Fig. 1.
Typical dose distribution (in colowash from 5 to 45 Gy) for axial, sagittal, and coronal views.
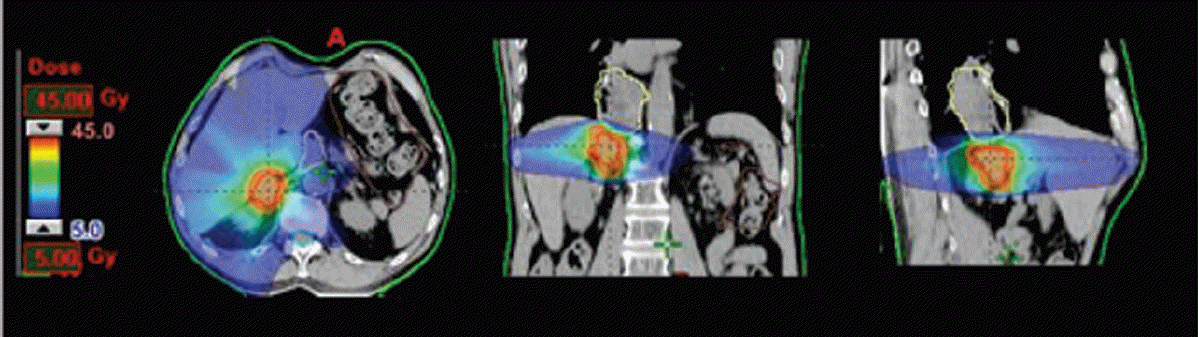
Fig. 2.
Average dose volume histograms (blue line) and interpatient variability at 1 standard deviation (red lines) for target volumes and organs at risk.
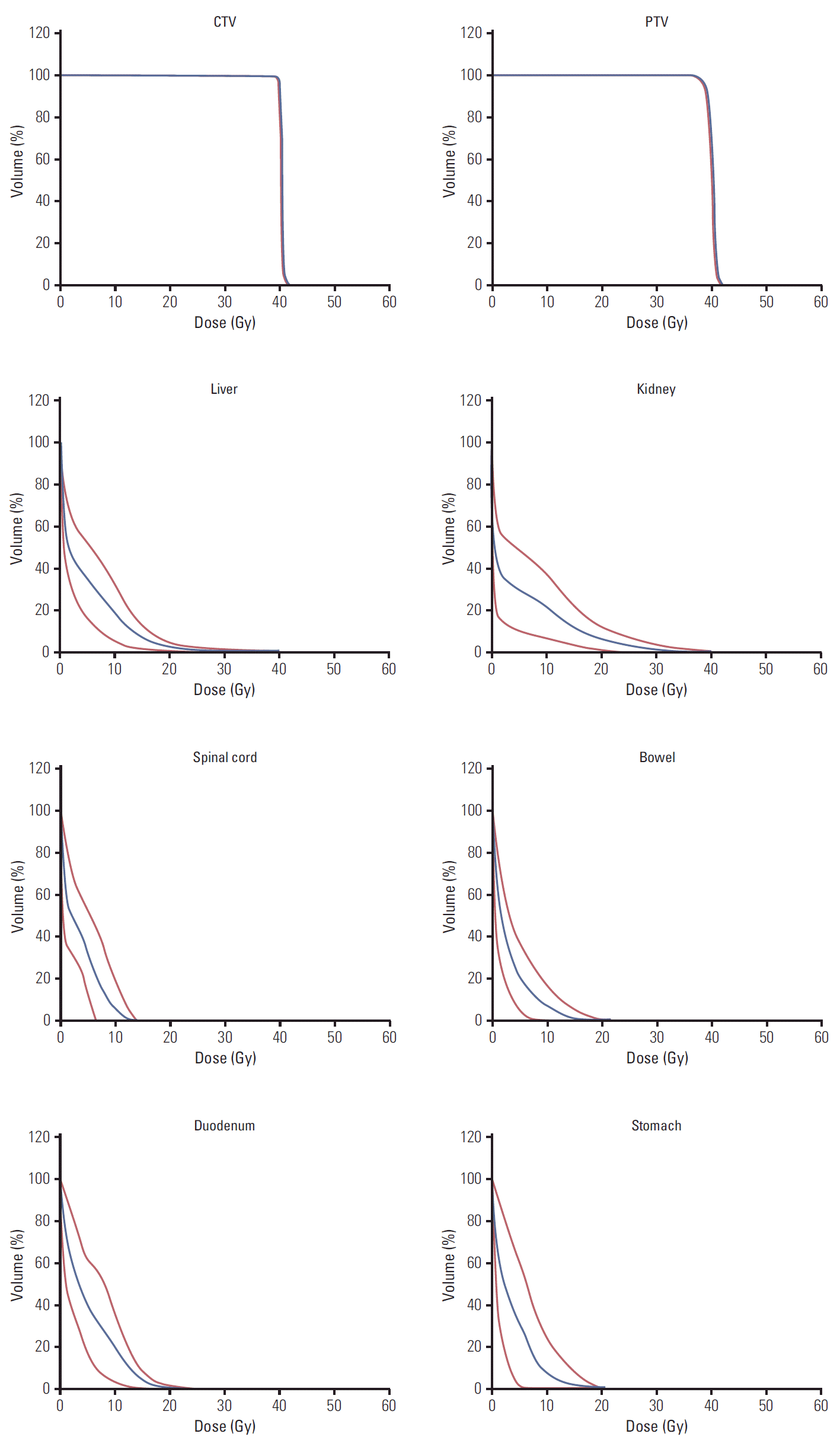
Table 1.
Summary of the patients’ characteristics
Table 2.
Summary of the quantitative analysis of the dosimetric findings from the treatment plans
Table 3.
Comparative summary of the studies reporting on SBRT treatment of adrenal gland metastases
| Author | No. of patients | Histology | Dose (Gy)/Fractionation | Median/Mean follow-up (mo) | Local control (%) | Overall survival (%) |
|---|---|---|---|---|---|---|
| Katoh et al. (2008) [22] | 8 | Miscellaneous | 30-48/8 | 16 | 1 Yr: 100 | 1 Yr: 78 |
| 2 Yr: 100 | - | |||||
| Chawla et al. (2009) [21] | 30 | Miscellaneous | 16-50/4-10 | 9.8 | 1 Yr: 55 | 1 Yr: 44 |
| 2 Yr: 27 | 2 Yr: 25 | |||||
| Torok et al. (2011) [23] | 7 | Miscellaneous | 10-36/3 | 14 | 1 Yr: 63 | - |
| Oshiro et al. (2011) [3] | 11 | Lung | 30-60/1-27 | 10.1 | 6 Mo: 97.4 | 1 Yr: 55.6 |
| - | 2 Yr: 33.4 | |||||
| Holy et al. (2011) [24] | 18 | Lung | 20-40/5 | 12 | 1 Yr: 94.4 | - |
| 2 Yr: 78.7 | - | |||||
| Casamassima et al. (2012) [20] | 48 | Miscellaneous | 21-54/3 | 16.2 | 1 Yr: 90 | 1 Yr: 39.7 |
| 2 Yr: 90 | 2 Yr: 14.5 | |||||
| Ahmed et al. (2013) [25] | 9 | Lung | 20-37.5/5 | 7.3 | 1 Yr: 44 | 1 Yr: 62.9 |
| 2 Yr: 44 | - | |||||
| Romero Arenas et al. (2014) [26] | 13 | Miscellaneous | 33.7-60/5 | 12.3 | Crude: 100 | 1 Yr: 62.9 |
| This study | 47 | Miscellaneous | 40/4 | 11.3 | 1 Yr: 65.5 | 1 Yr: 87.6 |
| 2 Yr: 40.1 | 2 Yr: 87.6 |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download