Abstract
Purpose
This study evaluated the benefits of adjuvant chemotherapy on elderly patients with advanced gastric cancer (AGC) using meta-analysis of well-designed randomized controlled clinical studies.
Materials and Methods
PubMed, Embase, and Cochrane were searched to retrieve clinical studies evaluating the benefits of adjuvant chemotherapy in the elderly with AGC. Hazards ratios (HRs) with 95% confidence intervals (CIs) were pooled across studies using a fixed-effects model.
Results
Two studies were included in this meta-analysis to estimate HR for the overall survival (OS), and relapse-free survival (RFS) between adjuvant chemotherapy and surgery in elderly and non-elderly patients. HR for OS in the elderly and non-elderly was 0.745 (95% CI, 0.552 to 1.006, p=0.055) and 0.636 (95% CI, 0.522 to 0.776; p < 0.001), respectively, which showed no heterogeneity regarding HR between the two groups (pinteraction=0.389). HR for RFS in the elderly and non-elderly was 0.613 (95% CI, 0.466 to 0.806; p < 0.001) and 0.633 (95% CI, 0.533 to 0.753; p < 0.001), respectively (pinteraction=0.846).
The incidence of gastric cancer in elderly people has been increasing with the expanded life span due to advanced medical science [1,2]. Elderly patients account for more than 50% of total gastric cancer development in Korea [2]. Clinically, most elderly patients could not complete all the planned adjuvant chemotherapy cycles, and a large proportion of patients stop chemotherapy for many reasons, including toxicity [3]. In subgroup analysis for the elderly patients, the benefit of adjuvant chemotherapy was statistically insignificant. This insignificance may result from the low statistical power or the ineffectiveness of adjuvant chemotherapy per se. Therefore, it is important to establish evidence-based guidelines of the adjuvant chemotherapy for the elderly with resectable advanced gastric cancer (AGC). Randomized controlled double blind studies are considered asthe most significant level of evidence for establishing a clinical guideline [4-7]. Recently, meta-analysis of randomized controlled double blind studies were shown to provide more concrete evidence for establishing clinical guidelines. Therefore, this study performed meta-analysis of the survival benefit of adjuvant chemotherapy to the elderly with randomized, controlled double blind studies.
This meta-analysis was exempt from ethical approval because it only involved published data and two individual studies had already received ethical approval.
The Medline (PubMed) (1968 to June 2015), Excerpta Medica database (1977 to June 2015), and the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (1953 to June 2015) were searched using common keywords related to adjuvant chemotherapy for elderly gastric cancer in randomized controlled trials (RCTs). The following keywords were selected for the literature search: (((stomach AND (neoplasm* OR cancer) OR gastric cancer) AND (adjuvant chemotherapy OR drug therapy) AND (gastrectomy OR surgical procedures) AND (survival OR mortality OR death)); AND (randomized controlled trials, OR randomized clinical trials)). Here, the keywords, “randomized controlled trials (RCT)” were used for the literature search because there were too many studies included without restriction, and high quality RCT-based evidence is needed.
RCTs that reported the effects of adjuvant chemotherapy on survival of the elderly patients (older than 65 years) were included and the results of these trials were compared with those of the control group who had received gastric surgery alone (D2 gastrectomy). Duplicated reports, reviews, meta-analysis articles, meeting abstracts, and studies without adequate information on the elderly were excluded. If multiple studies involved the same trials, only the complete ones were used in the final analysis. The results of article selection were compared and any discrepancies were resolved by discussion with the authors. The main outcome measure was the hazards ratios (HRs) with 95% confidence intervals (CIs) for overall survival (OS). Studies that did not investigate the treatment effect on elderly patients, even in subgroup analysis, were excluded.
The following data were extracted carefully and independently by three authors (S.-H.C., S.-I.G., and W.S.L.): the name of the first author, year of publication, study design, study location, study period, age, type of chemotherapy, type of surgery, OS, disease-free survival, and follow-up duration in the analysis. Any discrepancies in data extraction were discussed until consensus was reached.
This study mainly estimated the overall effects of adjuvant chemotherapy on the survival of elderly patients in randomized controlled double blind studies. In addition, the effects of adjuvant chemotherapy on the survival of non-elderly patients in the randomized controlled double blind studies were also evaluated. In addition, the benefits of adjuvant chemotherapy between elderly and non-elderly patients were compared.
The pooled HR with 95% CI were estimated based on the fixed-effects model for the treatment effects and relative risk (RR) with 95% CI on random-effects model for the toxicity. The Mantel-Haenszel method and DerSimonian and Laird method was used in the fixed-effects model and the random-effects model was used, respectively. The heterogeneity was estimated based on the clinical judgement and forest plot analysis. The Higgins I2 statistic was also used for heterogeneity estimation, which measures the percentage of total variation across the studies due to heterogeneity rather than chance. Heterogeneity was estimated using I2 values; the I2 values lie between 0% (no observed heterogeneity) and 100% (maximal heterogeneity). An I2 value was considered to indicate no heterogeneity (I2=0%-25%), moderate heterogeneity (I2=25%-50%), large heterogeneity (I2=50%-75%), and extreme heterogeneity (I2=75%-100%). The publication bias could not be estimated because two studies were included. The comprehensive meta-analysis ver. 2.0 (Biostat, Englewood, NJ) was used for statistical analysis.
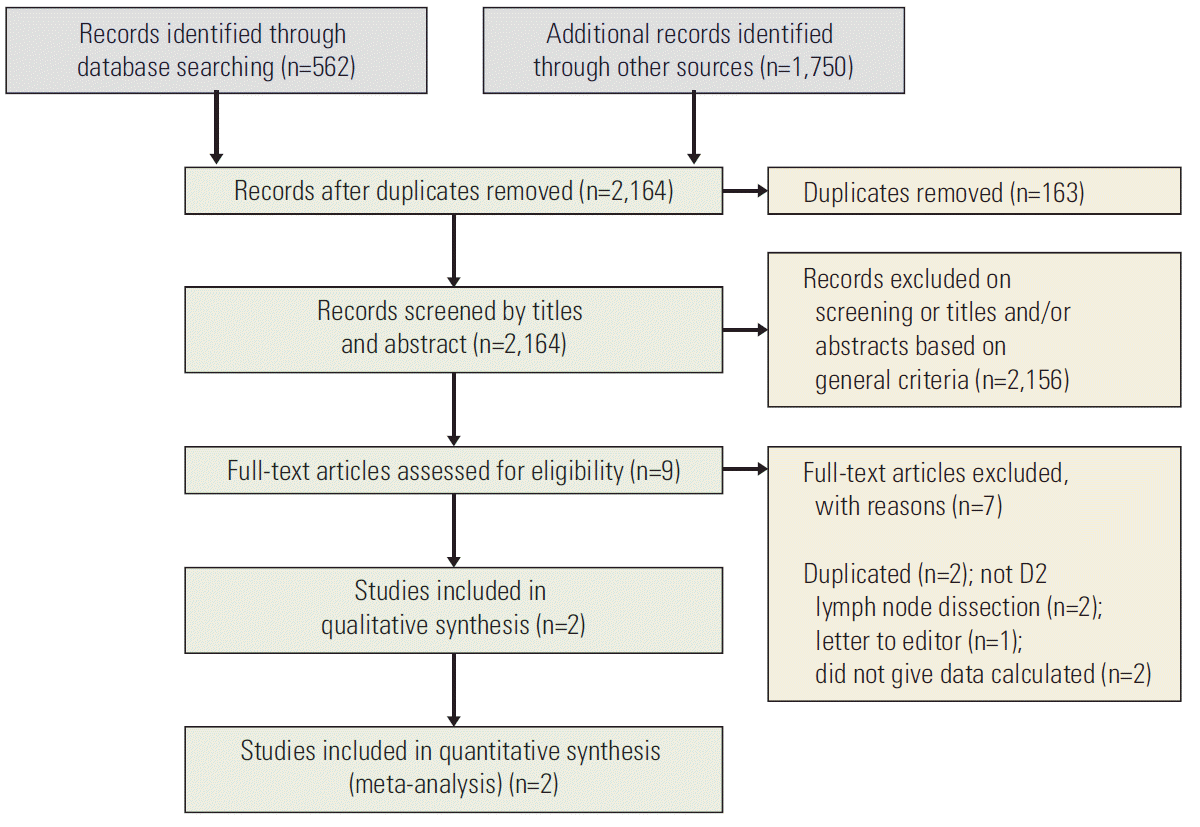
Fig. 1 presents a flow diagram showing the study selection; two articles were obtained after searching the databases. After exclusion of 149 duplicated articles and 2,156 articles that did not satisfy the selection criteria, the full texts of nine articles were reviewed. Among the nine articles [4-12], seven articles were excluded because two articles had identical populations and short-term study results [4,7], another two articles included patients who had received D1 or D3 lymph node dissection or D3 lymph node dissection [9,10]. One article was a letter to the editor [12]. The remaining two articles did not describe the results of the elderly patients [8,12]. Therefore, two RCTs were included in the final analysis.
The final analysis included 512 subjects out of a total of 2,069 who had enrolled in the two clinical trials. In the two studies, the subjects were distributed evenly; 1,045 in the control groups and 1,049 in the treatment groups. The elderly were defined as more 65 years old according to the subgroup designation of each study. Table 2 lists the general characteristics of the two articles included in the analysis. The articles selected were published from 2007 to 2014, spanning 7 years (four articles of the two studies). The countries in which the studies were conducted belonged to Asia (Korea and China, 1; Japan, 1). The median treatment and follow-up periods were 9 months and 5 years, respectively. The following two trials were reported in four articles with a 5-year follow-up. They had a homogenous control arm in that they were Asian and had received a D2 gastrectomy.
Overall, fixed-effects model meta-analysis of all selected studies (Fig. 2) showed that adjuvant chemotherapy had a significant positive influence on the OS, and relapse-free survival (RFS) of the patients with resectable AGC compared to D2 gastrectomy alone (OS: HR, 0.665; 95% CI, 0.565 to 0.784; p < 0.001; RFS: HR, 0.619; 95% CI, 0.536 to 0.714; p < 0.001; pinteraction=0.519). Heterogeneity was not observed across the selected studies (OS: p=0.936, I2 < 1%; RFS: p=0.421, I2 < 1%). These findings suggest that adjuvant chemotherapy improve the OS and RFS of the patients with AGC.
The two studies estimated the effects of adjuvant chemotherapy on both the elderly and non-elderly with AGC. The fixed-effects model meta-analysis showed that adjuvant chemotherapy also had a statistically significant positive influence on the OS of non-elderly patients with AGC compared to D2 gastrectomy alone (HR, 0.636; 95% CI, 0.522 to 0.776; p < 0.001), but not on the survival of the elderly patients (HR, 0.745; 95% CI, 0.552 to 1.006; p=0.055) (Fig. 3). Heterogeneity was not observed across the selected studies in each subgroup (non-elderly: p=0.651, I2 < 1%; elderly: p=0.731, I2 < 1%). On the other hand, there was no heterogeneity regarding HR between the elderly and non-elderly patients (pinteraction=0.389). These findings suggest that the survival benefit of adjuvant chemotherapy on the elderly is not large enough to reach statistical significance, even though HR < 1, and there was no heterogeneity regarding HR between the elderly and non-elderly patients.
In contrast to meta-analysis of the OS results, a fixedeffects model meta-analysis showed that adjuvant chemotherapy had a significant positive influence on the RFS of both the non-elderly and elderly patients with AGC compared to D2 gastrectomy alone (non-elderly: HR, 0.633; 95% CI, 0.533 to 0.753; p < 0.001; elderly: HR, 0.613; 95% CI, 0.466 to 0.806; p < 0.001; pinteraction=0.846) (Fig. 4). Heterogeneity was not observed across the selected studies in each subgroup (non-elderly: p=0.824, I2 < 1%; elderly: p=0.249, I2 < 1%). These findings suggest that adjuvant chemotherapy has a statistically significant impact on reducing the gastric cancer relapse after a gastrectomy in both elderly and non-elderly patients.
The incidence of grade 3 or 4 toxicity was reported in two trials. This study estimated RR for the grade 3 or 4 toxicity of adjuvant chemotherapy in the overall patients, because the data for the elderly were unavailable. Significant differences were observed between the two groups in terms of the incidence of leucopenia (RR, 12.362; 95% CI, 3.582 to 42.663; p < 0.001; I2=88.04%), and diarrhea (RR, 11.966; 95% CI, 2.830 to 50.596; p=0.001; I2 < 1%) (Fig. 5A and B). In addition, the incidence of chemo-regimen-specific toxicity (rash for S-1, and neuropathy for XELOX) was significantly different (RR, 4.474; 95% CI, 1.087 to 18.419; p < 0.038; I2=45.749%) (Fig. 5C). Unexpectedly, there were no significant differences between the two groups in terms of the incidence of thrombocytopenia (RR, 4.330; 95% CI, 0.704 to 26.649; p=0.114; I2=86.52%), vomiting (RR, 1.622; 95% CI, 0.986 to 2.668; p=0.057; I2=79.88%), and fatigue (RR, 2.582; 95% CI, 0.646 to 10.328; p=0.18; I2=81.604%) (Fig. 5D-F). On the other hand, meta-analysis revealed statistically significant heterogeneity in incidence of toxicities between the two studies (ACTS-DC and CLASSIC) (Fig. 5) in which different agents that may have different toxicity profiles were used in each study.
Meta-analysis of RCTs showed that the benefits of adjuvant chemotherapy were not statistically significant (p= 0.055), even though HR was less than 1 (HR, 0.745; 95% CI, 0.552 to 1.006), and there was no heterogeneity regarding HR between the elderly and non-elderly patients (pinteraction=0.389) in OS. This suggests that the survival benefit from adjuvant chemotherapy would be statistically significant if the population size increases. Here, what this study is pointing out is that the toxicities which most of patients suffer from would outstand the benefit of adjuvant chemotherapy to the elderly patients, even though the marginal survival benefits are, because lot of non-elderly patients who had received Xelox chemotherapy have been suffering the long-lasting neuropathy and other side effects even 2 years after chemotherapy. Unlike non-elderly patients, these side effects influence daytime activity and even ambulation elderly patients. The reduced daytime activity directly influence on the survival of elderly patients [13,14]. These finding suggests that the effects of adjuvant chemotherapy on OS may be different between elderly and non-elderly.
In this study, the data from subgroup analysis were used. This may cause some selection bias. On the other hand, the two studies analyzed appear to be very homogenous not just because I2 value was almost 0% but because all the patients had D2 lymph node dissection at very high quality hospitals. This suggest that the data from subgroup analysis may be valuable for the analysis. In addition, the analysis showed that, the benefits of adjuvant chemotherapy on RFS in the elderly patients were statistically significant (HR, 0.613; 95% CI, 0.466 to 0.806; p < 0.001). This finding suggests that adjuvant chemotherapy for elderly patients would have some anti-cancer effects in preventing cancer relapse itself, but this anticancer effect would not contribute directly to prolonging OS. This difference in survival benefit between RFS and OS is observed frequently in studies using cytotoxic chemotherapy [15-17] because of the chemotherapy-associated toxicities that may influence OS and post-chemotherapy quality of life of the elderly. This interpretation is supported by previous results of adjuvant therapy with fluorouracil and oxaliplatin in stage II elderly patients with colon cancer; the addition of oxaliplatin to fluorouracil with leucovorin (FL) decreases OS compared to FL in elderly patients in high risk stage II colon cancer, even though the addition of oxaliplatin appears to improve the RFS [15]. This is because the addition of oxaliplatin adversely influences the post-disease survival [15]. Therefore, meta-analysis was performed on the toxicities of adjuvant chemotherapies in this study; the results showed that grade 3 or 4 toxicities of adjuvant chemotherapies were much more frequent than those of surgery alone (Fig. 5).
Another possibility is that there is some difference in the clinicobiological characteristics of gastric cancer between elderly and non-elderly. Histologically, early gastric cancer of the elderly is predominantly the well-differentiated type, but the predominant histological type of AGC of the elderly is the mixed type, which is composed of well-differentiated adenocarcinoma in the superficial areas, and poorly-differentiated adenocarcinoma in the deeper areas [18,19]. These findings suggest that majority of elderly AGCs are principally well-differentiated adenocarcinomas, some of which have progressed to poorly-differentiated adenocarcinomas as they advance with time. In addition, comprehensive molecular characterization [20] by the Cancer Genome Atlas (TCGA) network also suggested that elderly gastric cancer has a more frequent subtype of microsatellite instable (MSI) gastric cancer (microsatellite instability-high cancer). These findings suggest that the characteristic features of elderly gastric cancer are different from those of the gastric cancer in younger patients [19,21-25]. Furthermore, MSI type gastric cancer is associated with 5-fluorouracil (5-FU) resistance [26-28]. These findings support the possibility that the benefit of 5-FU–based adjuvant chemotherapy to elderly patients is different from that to non-elderly patients.
This study had some limitations. First, the number of eligible studies was not large enough to show the small benefit of chemotherapy. However, considering that only two well-designed randomized studies were carried out over the recent 7 years (from 2007 to 2014), and that the subjects included in each study were not too small to detect the adjuvant chemotherapy benefit, the studies included in this study are sufficient for meta-analysis and its results would be reliable.
The second limitation is that the difference in effects between single chemotherapy and combination chemotherapy could not be determined clearly because the enrolled study is only two. In addition, the two studies showed that the OS benefit was similar (ACTS-GC study: 5-year OS, 71.7% for S-1 single adjuvant chemotherapy [S-1] vs. 61.1% for surgery alone [control]; CLASSIC study: 78% for XELOX combination adjuvant chemotherapy [XELOX] vs. 69% for control); they all showed an approximate 10% difference in OS between adjuvant chemotherapy following D2 gastrectomy and D2 gastrectomy alone. However, subgroup analysis showed that stage IIIB patients had more survival benefits from XELOX than did stage IIIA patients (the differences in 5-year OS between XELOX and control was 20% and 10% in stage IIIB and stage IIIA patients, respectively) [6] while in ACTS-GC study, the survival benefit of S-1 was smaller in stage IIIB patients than in stage IIIA patients (the differences in 5-year OS between S-1 and control was 5% and 10% in stage IIIB and stage IIIA patients, respectively) [5]. These findings suggest that there would be some differences in the effects of adjuvant chemotherapy between single and combination chemotherapy. In addition, in metastatic gastric cancer, combination chemotherapy is more effective in prolonging OS than single chemotherapy [26]. These findings also supports that there is some difference in effects between single chemotherapy and combination adjuvant chemotherapy following surgery. However, it is unknown whether this difference is also applied in the elderly patients because the characteristic features of the elderly gastric cancer are different from that of gastric cancer of younger patients [19,21-25], and the general condition of the elderly is too poor to tolerate combination chemotherapy. Therefore, further meta-analysis of the effects of single chemotherapy versus combination adjuvant chemotherapy is warranted.
The third limitation is that the benefit of adjuvant chemotherapy to the elderly with AGC was statistically insignificant, even though HR was less than 1 (HR, 0.745; 95% CI, 0.552 to 1.006), and no heterogeneity was observed regarding HR between the elderly and non-elderly patients. These findings, however, may explain the results that the survival benefit from adjuvant chemotherapy in elderly patients is smaller than that in nonelderly patients [29].
Finally, whether there would be some benefits of adjuvant chemotherapy to the elderly AGC cannot be ruled out completely, even though it was statistically insignificant. Therefore, randomized clinical trials are warranted to validate this result.
This meta-analysis suggests that the benefit of adjuvant chemotherapy to the elderly is still statistically insignificant, even though there was no statistically significant heterogeneity regarding HR between the elderly and non-elderly patients. Therefore, this statistical insignificant survival benefit from adjuvant chemotherapy suggests that a new treatment strategy is needed for the elderly patients with AGC considering the differences in the clinical and molecular characteristics of gastric cancer and the physical condition between elderly and non-elderly patients.
ACKNOWLEDGMENTS
This study was supported by a grant of the National R&D Program for Cancer Control, Ministry for Health, Welfare & Family Affairs, Republic of Korea (0820050).
References
1. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013; 45:1–14.

2. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Prediction of cancer incidence and mortality in Korea, 2013. Cancer Res Treat. 2013; 45:15–21.

3. Neugut AI, Matasar M, Wang X, McBride R, Jacobson JS, Tsai WY, et al. Duration of adjuvant chemotherapy for colon cancer and survival among the elderly. J Clin Oncol. 2006; 24:2368–75.

4. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007; 357:1810–20.

5. Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011; 29:4387–93.

6. Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014; 15:1389–96.

7. Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012; 379:315–21.

8. Bouche O, Ychou M, Burtin P, Bedenne L, Ducreux M, Lebreton G, et al. Adjuvant chemotherapy with 5-fluorouracil and cisplatin compared with surgery alone for gastric cancer: 7-year results of the FFCD randomized phase III trial (8801). Ann Oncol. 2005; 16:1488–97.
9. Nakajima T, Kinoshita T, Nashimoto A, Sairenji M, Yamaguchi T, Sakamoto J, et al. Randomized controlled trial of adjuvant uracil-tegafur versus surgery alone for serosa-negative, locally advanced gastric cancer. Br J Surg. 2007; 94:1468–76.

10. Cascinu S, Labianca R, Barone C, Santoro A, Carnaghi C, Cassano A, et al. Adjuvant treatment of high-risk, radically resected gastric cancer patients with 5-fluorouracil, leucovorin, cisplatin, and epidoxorubicin in a randomized controlled trial. J Natl Cancer Inst. 2007; 99:601–7.

11. Fazio N, Biffi R, Curigliano G, Lorizzo K, Zampino MG, de Braud F, et al. Re: Adjuvant treatment of high-risk, radically resected gastric cancer patients with 5-fluorouracil, leucovorin, cisplatin, and epidoxorubicin in a randomized controlled trial. J Natl Cancer Inst. 2007; 99:1345–6.

12. De Vita F, Giuliani F, Orditura M, Maiello E, Galizia G, Di Martino N, et al. Adjuvant chemotherapy with epirubicin, leucovorin, 5-fluorouracil and etoposide regimen in resected gastric cancer patients: a randomized phase III trial by the Gruppo Oncologico Italia Meridionale (GOIM 9602 Study). Ann Oncol. 2007; 18:1354–8.

13. Paganini-Hill A, Kawas CH, Corrada MM. Activities and mortality in the elderly: the Leisure World cohort study. J Gerontol A Biol Sci Med Sci. 2011; 66:559–67.

14. Molineux M. Social and productive activities in elderly people. Activity (occupation) is important for survival. BMJ. 2000; 320:184–5.
15. Tournigand C, Andre T, Bonnetain F, Chibaudel B, Lledo G, Hickish T, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol. 2012; 30:3353–60.

16. Huguenin P, Beer KT, Allal A, Rufibach K, Friedli C, Davis JB, et al. Concomitant cisplatin significantly improves locoregional control in advanced head and neck cancers treated with hyperfractionated radiotherapy. J Clin Oncol. 2004; 22:4665–73.

17. Hogberg T, Signorelli M, de Oliveira CF, Fossati R, Lissoni AA, Sorbe B, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer: results from two randomised studies. Eur J Cancer. 2010; 46:2422–31.
18. Inoshita N, Yanagisawa A, Arai T, Kitagawa T, Hirokawa K, Kato Y. Pathological characteristics of gastric carcinomas in the very old. Jpn J Cancer Res. 1998; 89:1087–92.

19. Arai T, Esaki Y, Inoshita N, Sawabe M, Kasahara I, Kuroiwa K, et al. Pathologic characteristics of gastric cancer in the elderly: a retrospective study of 994 surgical patients. Gastric Cancer. 2004; 7:154–9.

20. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014; 513:202–9.
21. Habu H, Endo M. Gastric cancer in elderly patients: results of surgical treatment. Hepatogastroenterology. 1989; 36:71–4.
22. Choi JY, Shim KN, Roh SH, Tae CH, Kim SE, Jung HK, et al. Clinicopathological characteristics of gastric cancer and survival improvement by surgical treatment in the elderly. Korean J Gastroenterol. 2011; 58:9–19.

23. Kitamura K, Yamaguchi T, Taniguchi H, Hagiwara A, Yamane T, Sawai K, et al. Clinicopathological characteristics of gastric cancer in the elderly. Br J Cancer. 1996; 73:798–802.

24. Edelman DS, Russin DJ, Wallack MK. Gastric cancer in the elderly. Am Surg. 1987; 53:170–3.
25. Ishigami S, Natsugoe S, Saihara T, Hokita S, Tokushige M, Watanabe T, et al. Clinical and pathologic features of early gastric cancer in elderly patients. Hepatogastroenterology. 1997; 44:1164–8.
26. Li LS, Morales JC, Veigl M, Sedwick D, Greer S, Meyers M, et al. DNA mismatch repair (MMR)-dependent 5-fluorouracil cytotoxicity and the potential for new therapeutic targets. Br J Pharmacol. 2009; 158:679–92.

27. Carethers JM, Smith EJ, Behling CA, Nguyen L, Tajima A, Doctolero RT, et al. Use of 5-fluorouracil and survival in patients with microsatellite-unstable colorectal cancer. Gastroenterology. 2004; 126:394–401.

Fig. 2.
Effects of adjuvant chemotherapy (CTX) for advanced gastric cancer on survival outcomes: overall survival (upper graph) and relapse-free survival (lower graph). Hazard ratios were analyzed using fixed effects model. Value 0-1 favors adjuvant chemotherapy. CI, confidence interval.
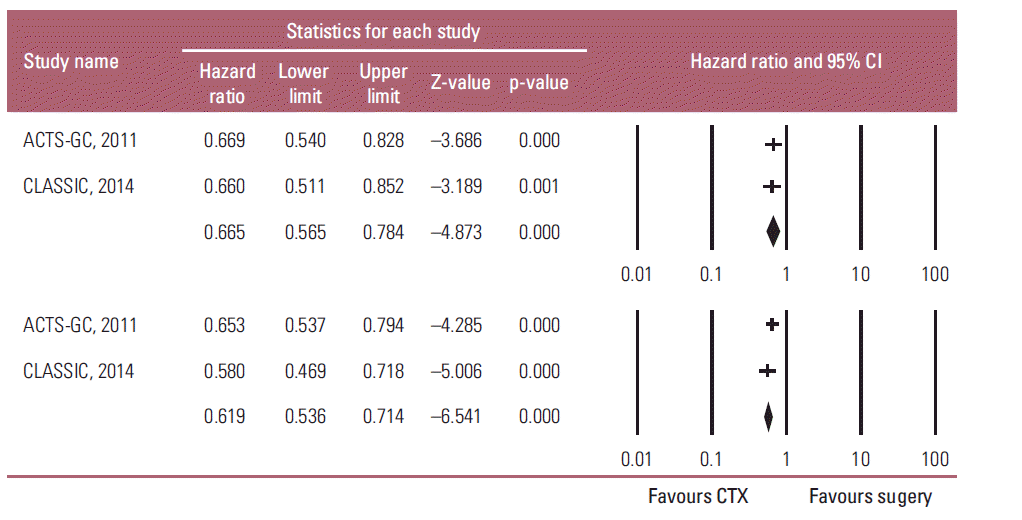
Fig. 3.
Subgroup analysis of the effects of adjuvant chemotherapy (CTX) on overall survival by age: non-elderly (upper graph) versus elderly patients (lower graph). Hazard ratios were analyzed with fixed effects model. Value 0-1 favors adjuvant CTX. CI, confidence interval.
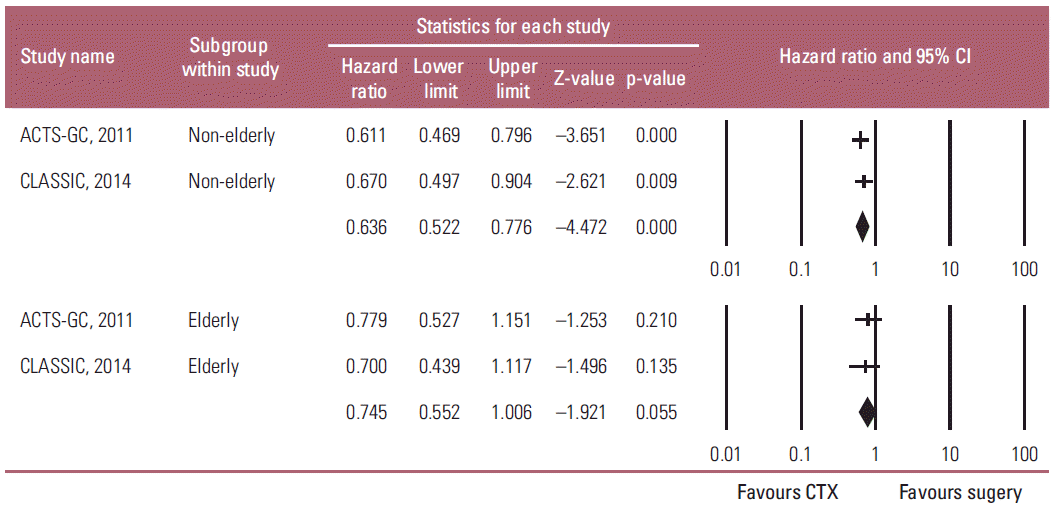
Fig. 4.
Subgroup analysis of the adjuvant chemotherapeutic effects on relapse-free survival by ages: non-elderly (upper graph) versus elderly patients (lower graph). Hazard ratios were analyzed using fixed effects model. Value 0-1 favors adjuvant chemotherapy (CTX). CI, confidence interval.
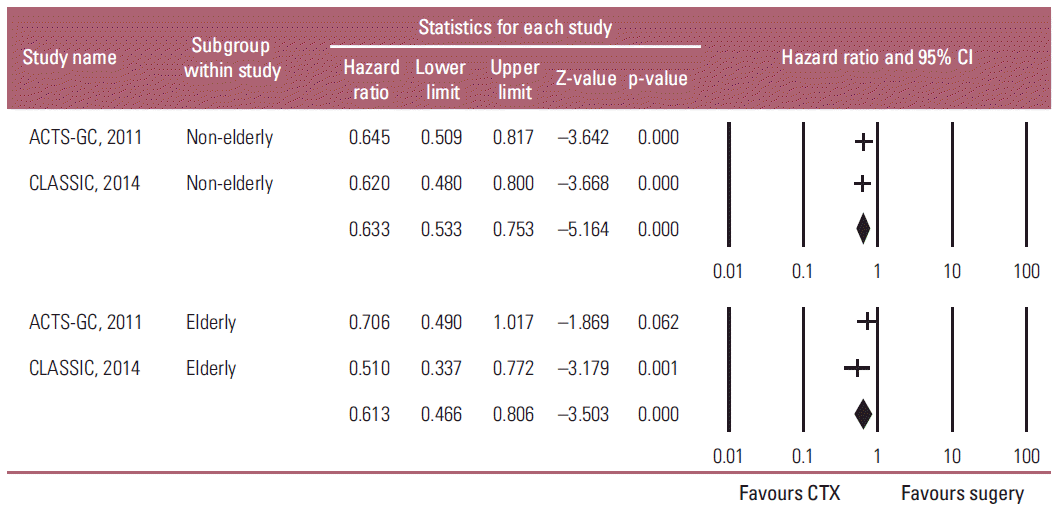
Fig. 5.
Toxic effects of adjuvant chemotherapy in terms of leukopenia (A), diarrhea (B), chemotherapy regimen-specific toxicities (C) (rash for S-1 in ACTS-GC study, and neuropathy for XELOX in CLASSIC study), thrombocytopenia (D), vomiting (E), and fatigue (F). Risk ratios were analyzed with random effects model. Value 0-1 favors adjuvant chemotherapy. CI, confidence interval; CTX, chemotherapy; XELOX, capecitabine plus oxaliplatin.
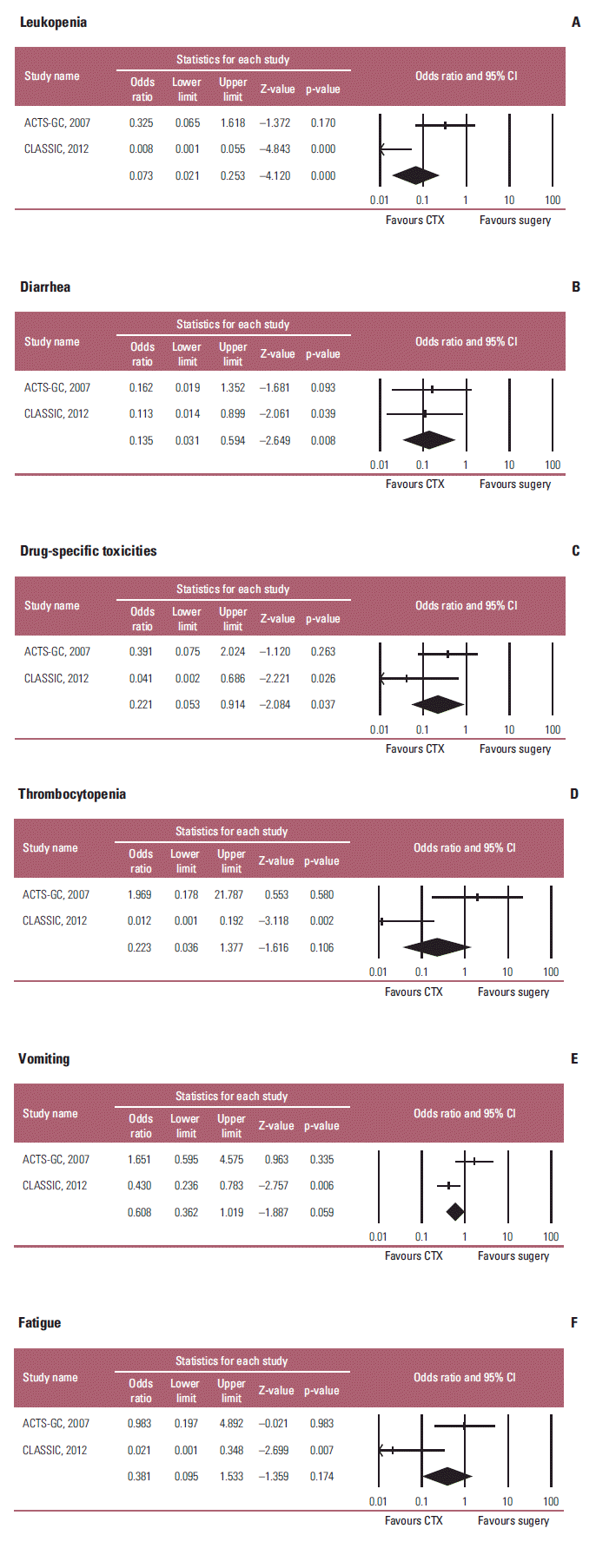
Table 1.
Results of assessment of risk of bias based on Cochrane's assessment of risk of bias
Table 2.
Characteristics of the studies included in meta-analysis




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download