Introduction
Materials and Methods
1. Data collection
2. Statistical analysis
Results
1. Physician characteristics
2. Patient and disease characteristics
Table 1.
| Variable | No. (%) |
|---|---|
| MGC patient | 198 (100) |
| Age at MGC diagnosis, mean±SD | 61.3±9.8 |
| Age group at MGC diagnosis (yr) | |
| 25-44 | 12 (6.1) |
| 45-54 | 31 (15.7) |
| 55-64 | 73 (36.9) |
| ≥ 65 | 82 (41.4) |
| Male | 146 (73.7) |
| Body mass indexa), mean±SD (kg/m2) | 21.4±2.6 |
| Smoking history | |
| Non-smoker | 83 (41.9) |
| Current smoker | 38 (19.2) |
| Former smoker | 55 (27.8) |
| Unknown | 22 (11.1) |
| Alcohol consumption | |
| No alcohol use | 68 (34.3) |
| Light to moderate | 94 (47.5) |
| Heavy | 18 (9.1) |
| Unknown | 18 (9.1) |
| History of Helicobacter pylori infection | 8 (4.0) |
| Family history of gastric cancer | 13 (6.6) |
| Charlson comorbidity index (CCI)b), mean±SD | 0.4±0.6 |
| Common comorbidity | |
| Chronic atrophic gastritis | 45 (22.7) |
| Cardiovascular disease | 37 (18.7) |
| Intestinal metaplasia | 28 (14.1) |
| Diabetes without chronic complicationsc) | 24 (12.1) |
| Chronic obstructive pulmonary diseasec) | 16 (8.1) |
| Peptic ulcer diseasec) | 15 (7.6) |
| Stage IV at MGC diagnosis | 193 (97.5) |
| Disease classification at MGC diagnosis | |
| Intestinal | 48 (24.2) |
| Diffuse | 72 (36.4) |
| Mixed | 13 (6.6) |
| Unknown | 65 (32.8) |
| Tumor location | |
| Antrum and pylorus | 102 (51.5) |
| Fundus and corpus | 48 (24.2) |
| Gastric cardia | 25 (12.6) |
| Esophagogastric junction | 11 (5.6) |
| Whole stomach | 7 (3.5) |
| Other | 1 (0.5) |
| Unknown | 4 (2) |
| Metastatic site | |
| Peritoneum | 106 (53.5) |
| Lymph nodes | 94 (47.5) |
| Liver | 77 (38.9) |
| Bone | 22 (11.1) |
| Lung | 9 (4.5) |
| Other | 9 (4.5) |
| Tested for HER2/neu gene expressiond) | 84 (42.4) |
| HER2 positive | 8 (9.5) |
| HER2 negative | 75 (89.3) |
| HER2 status unknown | 1 (1.2) |
MGC, metastatic and/or locally recurrent, unresectable gastric cancer; SD, standard deviation; HER2, human epidermal growth factor receptor 2.
a) Patients with weight less than 20 kg (n=1) were assumed to have the population average weight of 58 kg,
3. Disease and tumor characteristics
4. Treatment patterns
1) First-line therapy
Table 2.
| Variable | No. (%) |
|---|---|
| MGC patient | 198 (100) |
| Patient who received first-line chemotherapy treatment | 198 (100) |
| Patient who received second-line chemotherapy treatment | 159 (80.3) |
| Patient who received third-line chemotherapy treatment | 46 (23.2) |
| Patient who received BSC only after first-line | 39 (19.7) |
| ECOG PS score of first-line patientsa) | |
| 0: Asymptomatic | 25 (12.6) |
| 1: Symptomatic but completely ambulatory | 151 (76.3) |
| 2: Symptomatic, < 50% in bed during the day | 19 (9.6) |
| 4: Bedbound | 2 (1.0) |
| Unknown | 1 (0.5) |
| First-line regimenb) | |
| Fluoropyrimidine+platinum (+/- leucovorin) | 120 (60.6) |
| Capecitabine+platinum | 29 (14.6) |
| Single-agent fluoropyrimidine (+/- leucovorin) | 38 (19.2) |
| Reason for initiating second-line therapy | 159 (100) |
| Tumor progression | 152 (95.6) |
| Toxicity of first-line therapy | 7 (4.4) |
| ECOG PS score of second-line patientsa) | 159 (100) |
| 0: Asymptomatic | 16 (10.1) |
| 1: Symptomatic but completely ambulatory | 105 (66.0) |
| 2: Symptomatic, < 50% in bed during the day | 35 (22.0) |
| Unknown | 3 (1.9) |
| Second-line regimenb) | 159 (100) |
| Single agent fluoropyrimidine (+/- leucovorin) | 35 (22.0) |
| S-1 | 11 (6.9) |
| Capecitabine | 9 (5.7) |
| 5-FU | 7 (4.4) |
| Irinotecan+platinum and/or fluoropyrimidine (+/- leucovorin) | 31 (19.5) |
| Irinotecan, 5-FU, leucovorin | 15 (9.4) |
| Irinotecan, 5-FU | 10 (6.3) |
| Fluoropyrimidine+platinum agent (+/- leucovorin) | 21 (13.2) |
| Capecitabine+platinum agent | 9 (5.7) |
| 5-FU+platinum agent | 7 (4.4) |
| S-1+platinum agent | 5 (3.1) |
| Single-agent taxane | 13 (8.2) |
| Docetaxel | 9 (5.7) |
| Otherc) | 59 (37.1) |
| ECOG score of third-line patient | 46 (100) |
| 0: Asymptomatic | 5 (10.9) |
| 1: Symptomatic but completely ambulatory | 25 (54.3) |
| 2: Symptomatic, < 50% in bed during the day | 16 (34.8) |
| Unknown | 0 |
| Third-line regimen | |
| Single-agent fluoropyrimidine (+/- leucovorin) | 12 (26.1) |
| Fluoropyrimidine+platinum agent (+/- leucovorin) | 11 (23.9) |
| Single-agent taxane | 9 (19.6) |
| Irinotecan+platinum and/or fluoropyrimidine (+/- leucovorin) | 7 (15.2) |
| Other | 7 (15.2) |
MGC, metastatic and/or locally recurrent, unresectable gastric cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; 5-FU, 5-fluorouracil.
2) Second-line therapy and BSC
Table 3.
| Variable | First-line followed by BSC (n=39) | First-line followed by second-line (n=159) | p-valuea) |
|---|---|---|---|
| Age at MGC diagnosis (yr) | |||
| Years | 64.5±9.3 | 60.5±9.8 | 0.012* |
| Median (Q1-Q3) | 66 (60-71) | 62 (55-67) | |
| Distribution (yr) | |||
| 25-34 | 1 (2.6) | 3 (1.9) | > 0.990 |
| 35-44 | 0 | 8 (5.0) | 0.360 |
| 45-54 | 5 (12.8) | 26 (16.4) | 0.587 |
| 55-64 | 11 (28.2) | 62 (39.0) | 0.211 |
| ≥ 65 | 22 (56.4) | 60 (37.7) | 0.034* |
| Male | 31 (79.5) | 115 (72.3) | 0.363 |
| Ethnicity | |||
| East Asian | 39 (100) | 159 (100) | > 0.990 |
| BMI (kg/m2)b) | 21.8±2.8 | 21.3±2.5 | 0.121 |
| Smoking history | |||
| Non-smoker | 18 (46.2) | 65 (40.9) | 0.550 |
| Current smoker | 9 (23.1) | 29 (18.2) | 0.492 |
| Former smoker | 11 (28.2) | 44 (27.7) | 0.947 |
| Unknown | 1 (2.6) | 21 (13.2) | 0.084 |
| Alcohol consumption | |||
| No alcohol use | 11 (28.2) | 57 (35.8) | 0.368 |
| Light to moderate | 19 (48.7) | 75 (47.2) | 0.862 |
| Heavy | 6 (15.4) | 12 (7.5) | 0.131 |
| Unknown | 3 (7.7) | 15 (9.4) | > 0.990 |
| Performance status (ECOG score)c) | |||
| 0: Asymptomatic | 1 (2.6) | 24 (15.1) | 0.033* |
| 1: Symptomatic but completely ambulatory | 31 (79.5) | 120 (75.5) | 0.597 |
| 2: Symptomatic, < 50% in bed during the day | 5 (12.8) | 14 (8.8) | 0.542 |
| 3: Symptomatic, > 50% in bed, but not bedbound | 0 | 0 | - |
| 4: Bedbound | 2 (5.1) | 0 | 0.038* |
| Unknown | 0 | 1 (0.6) | > 0.990 |
Values are presented as mean±standard deviation or number (%) unless otherwise indicated. BSC, best supportive care; MGC, metastatic and/or locally recurrent, unresectable gastric cancer; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group.
a) Chi-squared or Fisher exact tests for categorical variables, Wilcoxon rank sum tests for continuous variables. p-values of < 0.05 are indicated by an asterisk (*),
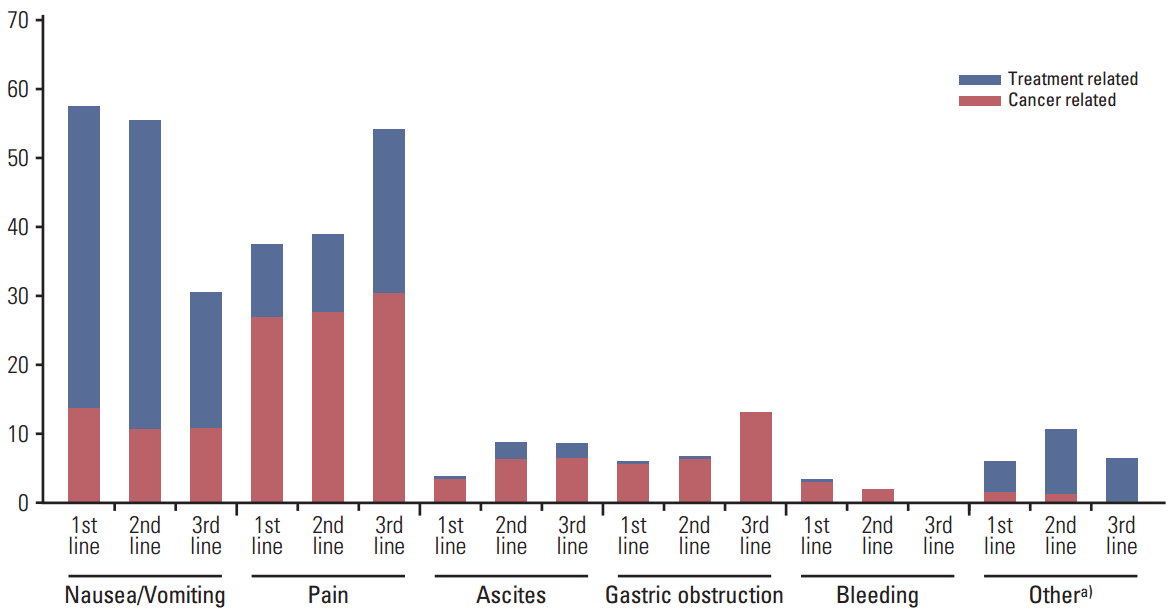
5. Physician-reported patient symptoms, supportive care, and healthcare resource use
Table 4.
| Variable | First-line (n=198) | Second-line (n=159) | BSC, no second-linea) (n=39) | Third-line (n=46) |
|---|---|---|---|---|
| Supportive care | ||||
| Antiemetics | 103 (52.0) | 71 (44.7) | 7 (17.9) | 21 (45.7) |
| Analgesics | 74 (37.4) | 58 (36.5) | 9 (23.1) | 13 (28.3) |
| Granulocyte-colony stimulating factors | 11 (5.6) | 13 (8.2) | 0 | 3 (6.5) |
| Diuretics | 9 (4.5) | 6 (3.8) | 1 (2.6) | 1 (2.2) |
| Antidepressants | 6 (3.0) | 4 (2.5) | 1 (2.6) | 0 |
| Erythropoiesis stimulating agents | 4 (2.0) | 2 (1.3) | 0 | 0 |
| GM-colony stimulating factors | 2 (1.0) | 0 | 0 | 0 |
| Narcotics | 0 | 1 (0.6) | 0 | 0 |
| Nutritional support | 27 (13.6) | 18 (11.3) | 4 (10.3) | 4 (8.7) |
| Inpatient hospitalization | ||||
| At least one stay | 71 (35.9) | 48 (30.2) | 13 (33.3) | 11 (23.9) |
| No. of visits/patient | 3.5±3.4 | 2.1±1.4 | 2.5±2.5 | 3.7±2.0 |
| Length of stay/hospitalization (day) | 8.2±9.4 | 9.1±11.3 | 14.3±15.6 | 10.2±12.5 |
| Main reasons for visit | ||||
| Chemotherapy infusion | 182 (73.4) | 68 (68.7) | 17 (53.1)b) | 23 (56.1) |
| Disease symptom management | 38 (15.3) | 21 (21.2) | 9 (28.1) | 11 (26.8) |
| Adverse events/toxicity | 19 (7.7) | 6 (6.1) | 2 (6.3) | 4 (9.8) |
| Pain management | 2 (0.8) | 2 (2.0) | 3 (9.4) | 3 (7.3) |
| Gastric cancer–related surgery | 6 (2.4) | 0 | 1 (3.1) | 0 |
| Regular monitoring | 0 | 2 (2.0) | 0 | 0 |
| Outpatient hospitalization (patients with information available) | 92 | 79 | 13 | 21 |
| At least one visit | 59 (64.1) | 25 (31.6) | 4 (30.8) | 7 (33.3) |
| Mean visits/patient | 3.2±4.3 | 2.5±3.3 | 1±0.0 | 2.7±2.5 |
| Hospice unit (patients with information available) | 34 | 29 | 5 | 10 |
| At least one stay | 1 (2.9) | 0 | 1 (20) | 2 (20.0) |
| Oncologist clinic (patients with information available) | 127 | 102 | 25 | 28 |
| At least one visit | 86 (67.7) | 60 (58.8) | 9 (36) | 10 (35.7) |
| No. of visits/patient | 3.9±3.3 | 3.0±3.6 | 5.1±5.2 | 2.8±1.9 |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download