Abstract
Purpose
Genetically engineered stem cells may be advantageous for gene therapy against various human cancers due to their inherent tumor-tropic properties. In this study, genetically engineered human neural stem cells (HB1.F3) expressing Escherichia coli cytosine deaminase (CD) (HB1.F3.CD) and human interferon-β (IFN-β) (HB1.F3.CD.IFN-β) were employed against lymph node–derived metastatic colorectal adenocarcinoma.
Materials and Methods
CD can convert a prodrug, 5-fluorocytosine (5-FC), to active 5-fluorouracil, which inhibits tumor growth through the inhibition of DNA synthesis,while IFN-β also strongly inhibits tumor growth by inducing the apoptotic process. In reverse transcription polymerase chain reaction analysis, we confirmed that HB1.F3.CD cells expressed the CD gene and HB1.F3.CD.IFN-β cells expressed both CD and IFN-β genes.
Results
In results of a modified trans-well migration assay, HB1.F3.CD and HB1.F3.CD.IFN-β cells selectively migrated toward SW-620, human lymph node–derived metastatic colorectal adenocarcinoma cells. The viability of SW-620 cells was significantly reduced when co-cultured with HB1.F3.CD or HB1.F3.CD.IFN-β cells in the presence of 5-FC. In addition, it was found that the tumor-tropic properties of these engineered human neural stem cells (hNSCs) were attributed to chemoattractant molecules including stromal cell-derived factor 1, c-Kit, urokinase receptor, urokinase-type plasminogen activator, and C-C chemokine receptor type 2 secreted by SW-620 cells. In a xenograft mouse model, treatment with hNSC resulted in significantly inhibited growth of the tumor mass without virulent effects on the animals.
Colorectal carcinoma (CRC) is a common cause of cancer death globally. The main cause of death from CRC is metastasis to other organs. Patients with localized and early stage CRC show relatively good prognosis after treatment; however, the prognosis is poor for patients with metastasis to multiple organs [1]. By the time of diagnosis, approximately 20% of CRC patients had already metastasized to other organs, particularly to the liver, the major site of metastasis for CRC [2]. Up to 15% to 20% of CRC patients present with liver metastasis, which in most cases (80%-90%) could not be removed by surgery. In addition, the incidence of lung metastasis is approximately 10% in CRC patients. In particular, lymph node metastasis is associated with metastasis to other organs such as liver and lung [3]. In addition, prognosis is poor in terms of overall mortality and recurrence for patients with lymph node metastasis of CRC. In most patients with CRC, there is no evidence of distant metastasis at the time of treatment, but the cancer has infiltrated deeply into the adjacent lymph nodes. In such cases, there is a high probability of tumor recurrence and metastasis locally or in distant organs such as liver and lungs [4]. For patients with CRC, the presence or absence of metastasis to regional lymph nodes is the most important determinant of diagnosis and survival [5].
A method known as gene-directed enzyme/prodrug therapy (GDEPT) could more selectively target human cancers and minimize the toxicity to normal tissues [6]. This system induces apoptosis of tumor cells by the bystander effect of enzymes which can convert an inactivate prodrug into an active drug. Cytosine deaminase (CD)/5-fluorocytosine (5-FC) is a well-known GDEPT system. The Escherichia coli CD can convert a prodrug, 5-FC, into the active drug, 5-fluorouracil (5-FU). The metabolite of 5-FU (fluorodeoxyuridine monophosphate) binds to the nucleotide-binding site of the thymidylate synthase and dNTP in tumor cells becomes imbalanced, which can lead to DNA damage and cell apoptosis [7]. This CD/5-FC system has been used in experimental treatments of cancers including breast and endometrial cancers, and successfully inhibited their growth [6]. The GDEPT systems appear to reduce the side effects compared with conventional methods for cancer treatment; however, problems still exist in creating a vehicle for effective delivery of exogenous enzymes to tumor cells [8]. In addition, interferon β (IFN-β) is a member of the type I IFN family, which can induce S-phase accumulation and apoptosis of tumor cells [9]. A high concentration of IFN-β in vitro could inhibit cancer cell growth; however, the in vivo therapeutic application is limited because of side effects when administered at high doses [10]. Because IFN-β has a short half-life, there is a limit in that sufficient concentration for cancer therapy cannot be reached [11].
Human neural stem cells (hNSCs) can be an effective delivery vehicle for gene therapy due to their inherent migratory ability to tumor sites [12]. To confirm this, HB1.F3 hNSCs obtained from fetal telencephalon were generated and immortalized using a retroviral vector encoding v-myc [13]. The immortalized hNSC (HB1.F3) was manipulated to generate genetically engineered neural stem cells expressing therapeutic genes, E. coli CD and/or human IFN-β to generate HB1.F3.CD and HB1.F3.CD.IFN-β cells [14]. In a previous study, we found that these genetically engineered hNSCs can migrate to various types of human cancers through interaction with several chemoattractant factors secreted by cancer cells, and a sufficient therapeutic effect of the stem cells was demonstrated by in vitro and in vivo studies [6,15-17].
In the current study, we further examined the question of whether these genetically engineered hNSCs might have a significant migrating capacity for selectively targeting colorectal adenocarcinoma metastasized to a lymph node, as well as therapeutic potential in colorectal cancer metastasized from a lymph node. We also identified the synergetic and therapeutic effects of chemotherapy consisting of the suicide gene/prodrug (CD/5-FC) coupled with immunotherapy (IFN-β) for the treatment of colorectal cancer that has metastasized from a lymph node using an in vitro co-culture system and an in vivo xenograft mouse model.
A human lymph node–derived metastatic colorectal adenocarcinoma cell line, SW-620, was purchased from Korean Cell Line Bank (KCLB, Seoul, Korea). Three types of engineered hNSCs, HB1.F3, HB1.F3.CD, and HB1.F3.CD.IFN-β, were provided by Dr. Seung U. Kim (University of British Columbia, Vancouver, BC, Canada) and used in this study. Primary human fibroblast cells (hFB) were used as a control for a transwell migration assay. All cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone Laboratories, Logan, UT) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS; Hyclone Laboratories), 10 U/mL penicillin and 100 μg/mL streptomycin (Cellgro Mediatech, Manassas, VA), 10 mM HEPES (Invitrogen Life Technologies, Carlsbad, CA), and anti-mycoplasmal agents (Invivogene, San Diego, CA) at 37°C in 5% CO2 and 95% air in a humidified cell incubator. The cells were trypsinized with 0.05% trypsin/0.02% ethylenediaminetetraacetic acid (PAA Laboratories, Dartmouth, MA).
Total mRNA extracts were prepared using TriZol Reagent (Invitrogen Life Technologies) and reverse transcribed from 1 μg of total mRNA into cDNA using M-MLV RT (iNtRON Biotechnology, Seongnam, Korea) following the manufacturer’s protocols. Polymerase chain reaction (PCR) was performed for amplification of the bacterial CD gene and human IFN-β genes from hNSCs using the respective primers. To confirm the expression of the chemoattractant factors by SW-620 cells, cDNAs of cancer cells were synthesized using sense and antisense primers including stromal cell-derived factor 1 (SDF-1), c-Kit, urokinase receptor (uPAR), urokinase-type plasminogen activator (uPA), and C-C chemokine receptor type 2 (CCR2). Human glyceraldehydes-3-phosphate dehydrogenase (GAPDH) gene was used as a positive control. PCR amplification of all genes, CD, IFN-β, and chemoattractant factors, was performed at 30 cycles. PCR products were analyzed on a 1.5% agarose gel pre-stained with ethidium bromide (Sigma-Aldrich, St. Louis, MO).
To examine the effect of 5-FU and 5-FC on lymph node-derived metastatic colorectal adenocarcinoma (SW-620) cells, cancer cells were seeded at 4,000 cells per well in 96-well plates and cultured in phenol free DMEM containing 5% charcoal-dextran treated FBS, 1% antibiotics (penicillin G and streptomycin), and 1% HEPES. After 24-hour incubation, the wells were treated with 5-FU or 5-FC diluted to different concentrations in phosphate buffered saline (PBS; 100, 200, 300, 400, and 500 μg/mL) for 3 days. To confirm a therapeutic effect of engineered hNSCs (HB1.F3, HB1.F3.CD, and HB1.F3.CD.IFN-β), the cancer cells were seeded in the same media (4,000 cells per well). After 24-hour incubation, engineered hNSCs were added to the cancer cell cultures, followed by incubation for 24 hours. Then, 5-FC diluted with PBS was added to each well followed by incubation for 3 days. Following treatment with 5-FU and 5-FC, the 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed for measurement of the cancer cell viability. MTT solution (10 μL; 5 mg/mL) was added to each well followed by incubation for 4 hours at 37°C. Supernatants were removed and 100 μL of dimethylsulfoxide (99.0%, Junsei Chemical, Tokyo, Japan) was added to each well to dissolve the resultant formazan crystals. Finally, optical densities were measured at 540 nm using an enzyme-linked immunosorbent assay reader (Epoch, BioTek, Winooski, VT). MTT assays were performed in triplicate.
To examine whether engineered hNSCs can migrate to lymph node–derived metastatic colorectal adenocarcinoma, SW-620 cells (1×105 cells per well) and human dermal fibroblast cells (a control, 1×105 cells per well) were plated in a 24-well plate containing 1% CD-FBS phenol free DMEM medium with incubation for 24 hours. The bottom surface of transwell plates (0.4 μm, BD Biosciences, Dickinson, Franklin Lakes, NJ) coated the with fibronectin (250 μg/mL, Sigma-Aldrich) was placed in the 24-well plates and CM-Dil (Invitrogen Life Technologies) pre-stained engineered hNSCs were plated in the upper chambers of the transwell plates at a density of 1×105 cells per well in the same condition medium and cultured for 24 hours at 37°C. After washing the lower chamber, the cells were fixed with 10% formalin solution (Sigma-Aldrich) for 10 minutes and permeabilized using 100% cold-methanol (Sigma-Aldrich) for 10 minutes. Then, DAPI (4',6-diamidino-2-phenylindole, Invitrogen Life Technologies) was added to the lower chamber at 300 nM and the plates were incubated for 10 minutes at 37°C followed by washing with PBS. Cells stained with CM-Dil and DAPI were examined by fluorescence microscopy (IX-73 Inverted Microscopy, Olympus, Tokyo, Japan) and counted by Cell Sense Dimension.
BALB/c nude female mice (4-week-old) were purchased from Daehan BioLink (Eumseong, Korea) and the experiments were performed according to the protocol (CBNUA-825-15-01) approved by the Animal Care Committee of Chungbuk National University. The room was kept at 24°C under a 12-hour light-dark cycle. The mice were acclimatized for at least 1 week prior to the start of experiments. To make animal models xenografted with lymph node–derived metastatic colorectal adenocarcinoma cells, SW-620 cells (1×106) were mixed with Matrigel (BD Biosciences, Bedford, MA) at 1:1 volume ratio of Matrigel to PBS in 100 μL and injected subcutaneously into the back of the mice. Mice were monitored for tumor growth every 3 days and the tumor volumes were measured using a caliper and expressed as length×width×height×0.5236 (mm3).
To confirm the therapeutic effect of engineered hNSCs in a xenograft model of lymph node-derived metastatic colorectal adenocarcinoma, the mice were divided into five experimental groups; group 1 was a negative control group treated with 0.85% PBS instead of hNSCs and 5-FC. Group 2 was treated with 5-FC (500 mg/kg/day) only, while group 3 was treated HB1.F3 (therapeutic gene is not expressed). Group 4 was treated with HB1.F3.CD in the presence of 5-FC, while group 5 was treated with HB1.F3.CD.IFN-β in the presence of 5-FC (500 mg/kg/day). This animal study was performed for 21 days after hNSCs injections. When tumor volume reached 500 mm3, CM-Dil pre-labeled hNSCs (4×106 cells per mouse) were injected subcutaneously adjacent to the tumor mass (group 3, HB1.F3; group 4, HB1.F3.CD; group 5, HB1.F3.CD.IFN-β). hNSCs were injected on the first day of each week. Two days after the injection of hNSCs, group 1 received intraperitoneal injections of PBS (400 μL), while groups 2, 3, 4, and 5 received intraperitoneal injections of 5-FC (500 mg/kg/day in 400 μL PBS) every day for 21 days. At 24 hours after the last 5-FC treatment, the mice were euthanized and tumor masses were harvested for molecular analysis.
For immunohistochemical analysis, tumor masses excised from the mice were washed with PBS and fixed in 10% formalin (OCI, Seoul, Korea). A fixed mass was sliced 5-mm-thick, dehydrated, and embedded in paraffin. Paraffin blocks were cut into 5-μm-thick sections. After drying the tissue slide, antigen retrieval was performed by boiling the slides for 10 minutes in a microwave using 0.01 M citrate buffer, and the slides were treated sequentially with 0.3% H2O2, blocking buffer, and primary antibodies. The primary antibody was a mouse monoclonal antibody against proliferating cell nuclear antigen (1:100, Abcam). For detection, biotinylated mouse IgG (1:1,000, Vector Laboratories Inc., Burlingame, CA) was used as a secondary antibody. The expression of each protein in a section of tumor mass was confirmed using a microscope (IX-73 inverted microscopy) for digital photography.
To examine the distribution of hNSCs in the tumor mass, the cells were previously stained with CM-Dil, according to the manufacturer’s protocol before injection. Tissue slides of tumor sections were hydrated and fixed in 10% formalin for 10 minutes, followed by washing with PBS, and then treatment with DAPI solution (600 nM) for 10 minutes at room temperature. Slides were mounted with coverslips and observed under a fluorescence microscope (IX-73 inverted microscopy).
In vitro and ex vivo experiments were performed at least three times to ensure consistent results. Graphpad Prism software (San Diego, CA) was used for statistical analysis of all data. In vitro and in vivo data were represented as the mean±standard error of the mean. Two-way analysis of variance (ANOVA) followed by post hoc Dunnett’s or Tukey’s multiple comparison test for three-pair comparisons or Student’s t test was used for data analysis, and p-values < 0.05 were considered statistically significant.
Expression of E. coli CD and human IFN-β genes in HB1.F3.CD or HB1.F3.CD.IFN-β cells was detected by reverse transcription polymerase chain reaction (RT-PCR). E. coli CD gene (559 bp) was expressed in both HB1.F3.CD and HB1.F3.CD.IFN-β cells, while human IFN-β gene (296 bp) was only detected in HB1.F3.CD.IFN-β cells (Fig. 1). The expression of GAPDH (351 bp) was used as an internal standard for RT-PCR study.
To examine the cytotoxic effects of 5-FC and 5-FU on lymph node–derived metastatic colorectal adenocarcinoma cells, SW-620 cells (4,000 cells per well) were seeded and treated with 5-FC or 5-FU at increasing concentrations (100 to 500 μg/mL), and the cell proliferation was then measured by MTT assay. Treatment with 5-FC for 3 days resulted in slightly decreased growth of SW-620 cells, while treatment with 5-FU resulted in significantly decreased cell proliferation (Fig. 2A). To confirm the cytotoxic activity of hNSCs, SW-620 cells were co-cultured with HB1.F3, HB1.F3.CD, or HB1.F3.CD.IFN-β in the presence of increasing concentrations of 5-FC (100 to 500 μg/mL). Compared with the control group (HB1.F3), cell proliferation of SW-620 cells was markedly decreased by HB1.F3.CD and HB1.F3.CD.IFN-β in the presence of 5-FC. As the amount of 5-FC was increased, the growth of cells was suppressed in a concentration dependent manner (Fig. 2B). HB1.F3.CD.IFN-β showed slightly greater anticancer effects than HB1.F3.CD, but without significance. In addition, microscopic observations of cell morphology in the culture dish confirmed that cell apoptosis and inhibition of cell proliferation were caused by hNSCs co-culture (Fig. 2C).
A modified transwell migration assay was further performed to measure the capacity of hNSCs to migrate toward lymph node–derived metastatic colorectal adenocarcinoma cells. SW-620 cells and hFBs (1×105 cells/mL) were seeded in a 24-well plate containing serum free media with incubation for 24 hours. In this study, CM-Dil labeled hNSCs, HB1.F3, HB1.F3.CD, or HB1.F3.CD.IFN-β, migrated towards SW-620 cells, as shown by fluorescence microscopy (Fig. 3A). The number of hNSCs that migrated to SW-620 or hFB was counted using fluorescence microscopy and the migratory ratio was calculated. The migratory capacity of hNSCs to SW-620 cells was significantly increased approximately 6 times compared to hFB cells (Fig. 3B). These results indicate that genetically engineered hNSCs have a tumor-tropic potential towards lymph node–derived metastatic colorectal adenocarcinoma cells. There was no significant difference in the migratory capacity among HB1.F3, HB1.F3.CD, and HB1.F3.CD.IFN-β (Fig. 3B). RT-PCR was performed to confirm expression of chemoattractant factors in SW-620 cells. The chemoattractant factors in SW-620 cells, c-Kit (570 bp), SDF-1a (263 bp), uPAR (63 bp), CCR2 (100 bp), and uPA (94 bp), were identified at the mRNA levels by RT-PCR (Fig. 3C).
A xenografted mouse model was used to examine the anticancer effect of engineered hNSCs via in vivo study (Fig. 4A). To establish a lymph node–derived metastatic colorectal adenocarcinoma xenograft model, SW-620 cells (1×106 cells per mouse) were injected subcutaneously into female BALB/c nude mice. During the experimental period, tumor masses were measured using digital calipers. Injection of hNSCs and intraperitoneal treatment with 5-FC were started when the volume of the tumor masses reached 500 mm3. From 21 days during the experimental period, the growth of the tumor mass was significantly suppressed by treatment with HB1.F3.CD or HB1.F3.CD.IFN-β in the presence of 5-FC prodrug (approximately 45%) compared to controls (PBS, 5-FC alone, or HB1.F3 alone) (Fig. 4B). Treatment with HB1.F3 cells alone, which do not express a therapeutic gene, did not result in suppression of the tumor mass in a xenografted mouse model. Based on these results genetically engineered hNSCs have therapeutic potential in lymph node metastatic colorectal cancer in vivo. However, HB1.F3.CD.IFN-β did not show an additional effect in terms of suppression of the tumor masses compared to HB1.F3.CD in the presence of 5-FC (Fig. 4B).
A fluorescence analysis was performed using CM-Dil prelabeled hNSCs injected near the tumor mass to confirm the migratory ability of hNSCs towards the tumor mass. We confirmed the presence of red fluorescent hNSCs on the surface and inside the tumor masses (Fig. 5). These results indicate that hNSCs could migrate towards tumor masses in vivo by recognition of chemoattractant ligand-receptor signals.
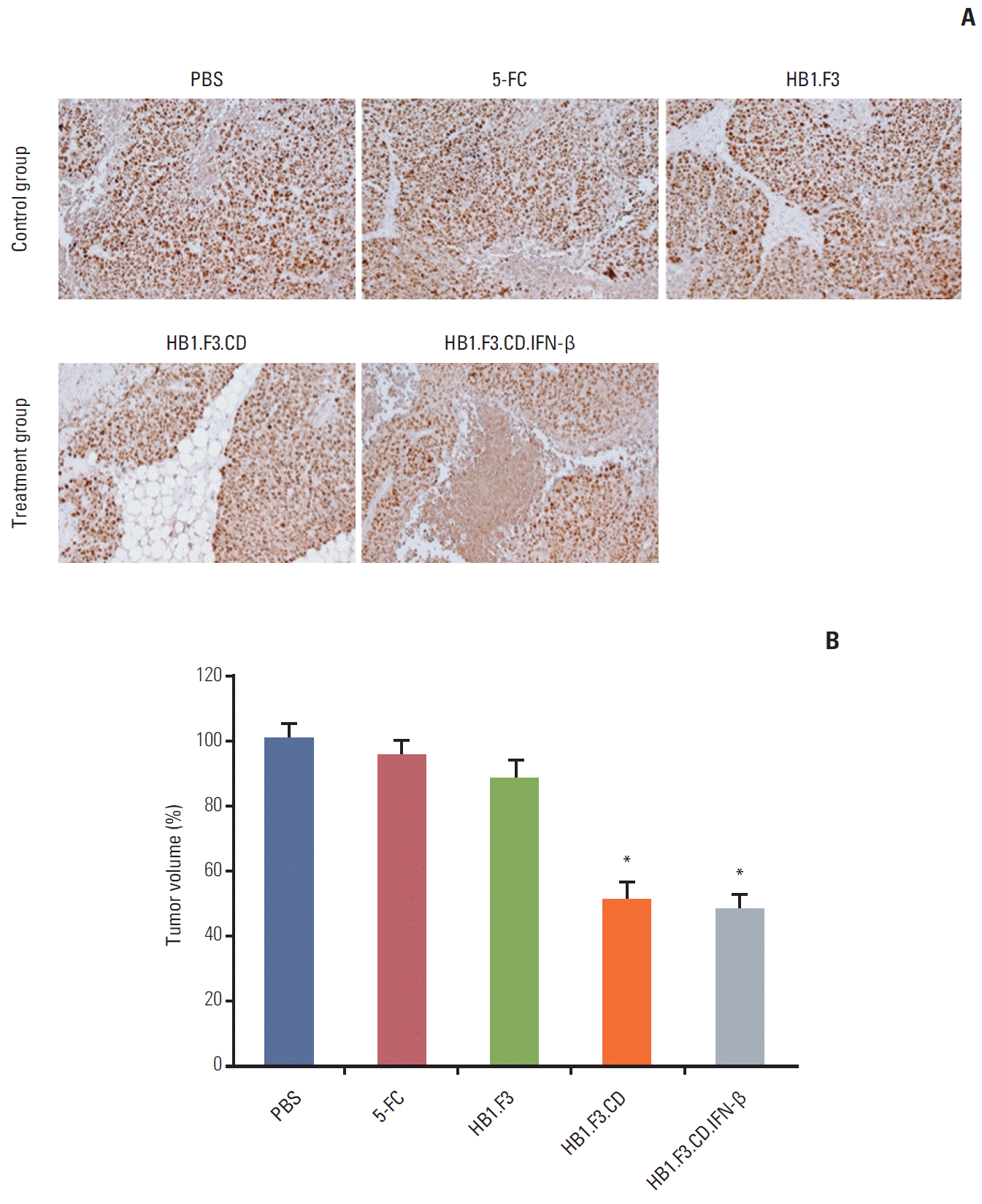
Immunohistochemical analysis was further performed to elucidate how the growth of tumor masses was inhibited by hNSCs in the presence of a prodrug. The results showed that the expression of proliferating cell nuclear antigen, a marker of proliferation, was significantly downregulated by treatment with HB1.F3.CD or HB1.F3.CD.IFN-β in the presence of 5-FC prodrug compared to the controls, PBS, 5-FC alone, or HB1.F3 alone (Fig. 6A). In addition, the positive stained cells of PCNA were counted in the tumor tissues using a microscope. The number of positive stained cells expressing PCNA was significantly reduced by treatment with hNSCs in the presence of 5-FC up to 50%, indicating that hNSCs can successfully activate 5-FC to 5-FU and inhibit the cell proliferation of lymph node–derived metastatic colorectal adenocarcinoma cells in a xenografted mouse model (Fig. 6B).
Colorectal cancer metastasizes to multiple organs through lymph nodes [18]. In the process of metastasis, lymph nodes act as a passage for movement of tumor cells to other organs [19]. In fact, metastasis of CRC to lymph nodes is a major factor in determining the survival rate and diagnosis after treatment [20]. Therefore, therapies targeting the metastasis of CRC to lymph nodes are needed. However, the conventional method of treatment for metastatic CRC has limitations in that surgical removal method has a relatively high recurrence rate and chemotherapy causes systemic side effects [21].
Therapeutic method using GDEPT as an alternative to conventional chemotherapy has many advantages against various types of cancers [22-24]. The system can convert non-toxic prodrugs into their active anticancer drugs. In particular, the CD/5-FC system has demonstrated a sufficient anticancer effect for several types of cancers including breast, brain, and prostate cancer [6,25,26]. In addition, the use of stem cells which can migrate to the cancer is an effective strategy for delivery of therapeutic genes to specific tumor regions.
Mesenchymal stem cells (MSCs) obtained from bone marrow or umbilical cord are a candidate for cell based therapy for specific cancer sites [27]. These cells could be genetically manipulated to express therapeutic genes, i.e., CD and/or IFN-β, for use as a delivery vehicle. They have the tumor tropic abilities to migrate by recognizing chemoattractant factors expressed by cancer cells. The proliferation of several cancer cell lines was suppressed by genetically engineered stem cells treated with 5-FC, a prodrug [28]. In addition, a better anticancer effect was confirmed when stem cells were engineered to express the IFN-β gene. These studies emphasized the importance of the delivery system for specific delivery of genes to enhance the selective antitumor effect. However, some research data indicate that MSCs promote tumor growth through interaction between tumor cells and MSCs, and increased the expression of tumor promoting factors which induced tumor cell proliferation and angiogenesis, as previously shown [29].
In this study, we confirmed that the effectiveness of these genetically engineered hNSCs was adequate for use in lymph node metastatic colorectal cancer therapy. The tumor tropic ability of HB1.F3.CD and HB1.F3.CD.IFN-β cells can be proven for selective targeting of human cancer cells, not for normal cells, through interaction with chemoattractant factors expressed by cancer cells which were carefully identified in this and other studies [30]. However, the specific molecular mechanism should be explained in further study.
This study employed genetically engineered hNSCs, which expressed E. coli CD and/or human IFN-β. HB1.F3, HB1.F3.CD, and HB1.F3.CD.IFN-β cells were further generated by retroviral transduction using an immortalized stem cell line. By combining the gene based prodrug therapy (5-FC/CD) and immunotherapy (IFN-β), a synergistic effect was expected for the treatment of lymph node metastatic colorectal cancer. In addition, the migratory efficacy of the engineered hNSCs towards lymph node–derived metastatic colorectal adenocarcinoma cells was confirmed using a modified trans-well assay. The engineered hNSCs migrated more efficiently towards SW-620 than normal human fibroblasts. The importance of the chemoattractant factors expressed by tumor cells was further determined using RT-PCR. SW-620 cells expressed several chemoattractant factors, including c-Kit, SDF-1a, uPAR, CCR2, and uPA, suggesting that these molecules might accelerate the migration of genetically engineered hNSCs towards the tumor cells for selective delivery of anticancer enzyme and/or cytokine by ligand-receptor signaling pathways. Further study is required to confirm the specific role(s) of these factors in tumor cell recognition and tumor tropism of engineered neural stem cells against human tumors.
In the current study, treatment with HB1.F3.CD and/or HB1.F3.CD.IFN-β in the presence of 5-FC resulted in significant suppression of lymph node–derived metastatic colorectal adenocarcinoma in both in vitro and in vivo studies. In a co-culture system, treatment with HB1.F3.CD or HB1.F3.CD. IFN-β in the presence of a prodrug, 5-FC resulted in decreased viability of SW-620 by 45%. In a xenografted mouse model, the tumor volume of injected lymph node-derived metastatic colorectal adenocarcinoma cells was significantly decreased by approximately 50% by treatment with hNSCs compared with control groups. In this study, in an in vivo model treatment with HB1.F3 in the presence of a prodrug or prodrug alone without hNSCs did not result in any alteration of tumor formation, suggesting that presence of both hNSCs and prodrug is required to suppress tumor formation of lymph node metastatic colorectal cancer.
In in vitro study, treatment with 5-FU had a greater effect on apoptotic induction and cell growth inhibition of SW-620 cells compared with the hNSCs/prodrug system, suggesting that there is a limitation in the enzyme activity (CD) of hNSCs to convert a prodrug, 5-FC, to active form, 5-FU. The prodrug conversion efficiency of genetically engineered hNSCs should be improved via enzymatic manipulation or reintroduction of therapeutic genes to adjust their expression levels. In our previous studies, a synergistic anticancer effect of HB1.F3.CD.IFN-β cells was observed [6,25]. However, a synergistic effect of IFN-β was not confirmed on lymph node metastatic colorectal cancer therapy in this study, implying that the response to CD/5-FC and/or IFN-β may vary in diverse types of cancer. In particular, SW-620 is a metastatic colorectal cancer cell line derived from lymph nodes, which may have resistance to cytokines. Based on this result, combination with another gene is required for the synergistic effect on human cancer therapy. IFN-β is a potent cytokine but has a low clinical effect owing to its short half-life, thus the secretion level of the functional form of IFN-β should be quantified from HB1.F3.CD or HB1.F3.CD.IFN-β cells into culture media using the enzyme-linked immunosorbent assay method in further study. In addition, development of a regulatory system for adjustment of neural stem cells to prevent possible off-target effect on normal tissues would be important.
In conclusion, the results of the current study suggest the potential for use of genetically engineered hNSCs expressing CD and/or IFN-β in treatment of lymph node metastatic colorectal cancer through their tumor-tropic capacity in the presence of a prodrug. It can be expected that this updated gene therapy using hNSCs would have clinical use in reinforcing the shortcomings of conventional anti-cancer treatment for lymph node metastatic colorectal cancer.
ACKNOWLEDGMENTS
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST) (2013R1A1A2059092). In addition, this work was supported by the Priority Research Centers Program through NRF funded by the Ministry of Education, Science and Technology (2015R1A6A1A04020885). The authors appreciate Ms. Jae-Rim Heo for her technical support in preparation of the manuscript.
References
1. Galandiuk S, Wieand HS, Moertel CG, Cha SS, Fitzgibbons RJ Jr, Pemberton JH, et al. Patterns of recurrence after curative resection of carcinoma of the colon and rectum. Surg Gynecol Obstet. 1992; 174:27–32.
2. Khattak MA, Martin HL, Beeke C, Price T, Carruthers S, Kim S, et al. Survival differences in patients with metastatic colorectal cancer and with single site metastatic disease at initial presentation: results from South Australian clinical registry for advanced colorectal cancer. Clin Colorectal Cancer. 2012; 11:247–54.

3. Dhar DK, Yoshimura H, Kinukawa N, Maruyama R, Tachibana M, Kohno H, et al. Metastatic lymph node size and colorectal cancer prognosis. J Am Coll Surg. 2005; 200:20–8.

4. Rahbari NN, Bork U, Motschall E, Thorlund K, Buchler MW, Koch M, et al. Molecular detection of tumor cells in regional lymph nodes is associated with disease recurrence and poor survival in node-negative colorectal cancer: a systematic review and meta-analysis. J Clin Oncol. 2012; 30:60–70.

5. Swanson RS, Compton CC, Stewart AK, Bland KI. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol. 2003; 10:65–71.

6. Yi BR, Hwang KA, Aboody KS, Jeung EB, Kim SU, Choi KC. Selective antitumor effect of neural stem cells expressing cytosine deaminase and interferon-beta against ductal breast cancer cells in cellular and xenograft models. Stem Cell Res. 2014; 12:36–48.

7. Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003; 3:330–8.

8. Freytag SO, Khil M, Stricker H, Peabody J, Menon M, DePeralta-Venturina M, et al. Phase I study of replication-competent adenovirus-mediated double suicide gene therapy for the treatment of locally recurrent prostate cancer. Cancer Res. 2002; 62:4968–76.
9. Garrison JI, Berens ME, Shapiro JR, Treasurywala S, Floyd-Smith G. Interferon-beta inhibits proliferation and progression through S phase of the cell cycle in five glioma cell lines. J Neurooncol. 1996; 30:213–23.

10. Ren C, Kumar S, Chanda D, Kallman L, Chen J, Mountz JD, et al. Cancer gene therapy using mesenchymal stem cells expressing interferon-beta in a mouse prostate cancer lung metastasis model. Gene Ther. 2008; 15:1446–53.
11. Le Page C, Genin P, Baines MG, Hiscott J. Interferon activation and innate immunity. Rev Immunogenet. 2000; 2:374–86.
12. Yi BR, Kim SU, Kim YB, Lee HJ, Cho MH, Choi KC. Antitumor effects of genetically engineered stem cells expressing yeast cytosine deaminase in lung cancer brain metastases via their tumor-tropic properties. Oncol Rep. 2012; 27:1823–8.

13. Kim SU, Nakagawa E, Hatori K, Nagai A, Lee MA, Bang JH. Production of immortalized human neural crest stem cells. Methods Mol Biol. 2002; 198:55–65.

14. Ito S, Natsume A, Shimato S, Ohno M, Kato T, Chansakul P, et al. Human neural stem cells transduced with IFN-beta and cytosine deaminase genes intensify bystander effect in experimental glioma. Cancer Gene Ther. 2010; 17:299–306.
15. Yi BR, Kim SU, Choi KC. Co-treatment with therapeutic neural stem cells expressing carboxyl esterase and CPT-11 inhibit growth of primary and metastatic lung cancers in mice. Oncotarget. 2014; 5:12835–48.

16. Yi BR, Hwang KA, Kang NH, Kim SU, Jeung EB, Kim HC, et al. Synergistic effects of genetically engineered stem cells expressing cytosine deaminase and interferon-beta via their tumor tropism to selectively target human hepatocarcinoma cells. Cancer Gene Ther. 2012; 19:644–51.
17. Yi BR, Kim SU, Choi KC. Synergistic effect of therapeutic stem cells expressing cytosine deaminase and interferon-beta via apoptotic pathway in the metastatic mouse model of breast cancer. Oncotarget. 2016; 7:5985–99.

18. Bockelman C, Engelmann BE, Kaprio T, Hansen TF, Glimelius B. Risk of recurrence in patients with colon cancer stage II and III: a systematic review and meta-analysis of recent literature. Acta Oncol. 2015; 54:5–16.
20. Fulmes M, Setrakian S, Raj PK, Bogard BM. Cancer biology and necrotic changes in metastatic lymph nodes and survival of colon cancer patients. Am J Surg. 2005; 189:364–8.

21. Guyot F, Faivre J, Manfredi S, Meny B, Bonithon-Kopp C, Bouvier AM. Time trends in the treatment and survival of recurrences from colorectal cancer. Ann Oncol. 2005; 16:756–61.

22. Yi C, Huang Y, Guo ZY, Wang SR. Antitumor effect of cytosine deaminase/5-fluorocytosine suicide gene therapy system mediated by Bifidobacterium infantis on melanoma. Acta Pharmacol Sin. 2005; 26:629–34.

23. Wang X, Ji C, Ma D, Zhao J, Hou M, Yu H, et al. Antitumor effects of cytosine deaminase and thymidine kinase fusion suicide gene under the control of mdr1 promoter in mdr1 positive leukemia cells. Leuk Lymphoma. 2007; 48:1600–9.

24. You MH, Kim WJ, Shim W, Lee SR, Lee G, Choi S, et al. Cytosine deaminase-producing human mesenchymal stem cells mediate an antitumor effect in a mouse xenograft model. J Gastroenterol Hepatol. 2009; 24:1393–400.

25. Yi BR, Hwang KA, Kim YB, Kim SU, Choi KC. Effects of genetically engineered stem cells expressing cytosine deaminase and interferon-beta or carboxyl esterase on the growth of LNCaP rrostate cancer cells. Int J Mol Sci. 2012; 13:12519–32.
26. Yi BR, Park MA, Lee HR, Kang NH, Choi KJ, Kim SU, et al. Suppression of the growth of human colorectal cancer cells by therapeutic stem cells expressing cytosine deaminase and interferon-beta via their tumor-tropic effect in cellular and xenograft mouse models. Mol Oncol. 2013; 7:543–54.
27. Nelson TJ, Martinez-Fernandez A, Yamada S, Ikeda Y, Perez-Terzic C, Terzic A. Induced pluripotent stem cells: advances to applications. Stem Cells Cloning. 2010; 3:29–37.
28. Kucerova L, Skolekova S, Demkova L, Bohovic R, Matuskova M. Long-term efficiency of mesenchymal stromal cell-mediated CD-MSC/5FC therapy in human melanoma xenograft model. Gene Ther. 2014; 21:874–87.

Fig. 1.
Expression of Escherichia coli cytosine deaminase (CD) and human interferon β (IFN-β) genes in genetically engineered human neural stem cells. Expression of introduced therapeutic genes, CD and IFN-β, was confirmed. After total RNA extraction from HB1.F3.CD and HB1.F3.CD.IFN-β cells, cDNAs were synthesized. These cDNAs were amplified by polymerase chain reaction (PCR), and PCR products were confirmed by gel electrophoresis. The transcripts of E. coli CD (559 bp) and human IFN-β (296 bp) were detected by PCR. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 351 bp) was used as an internal control.
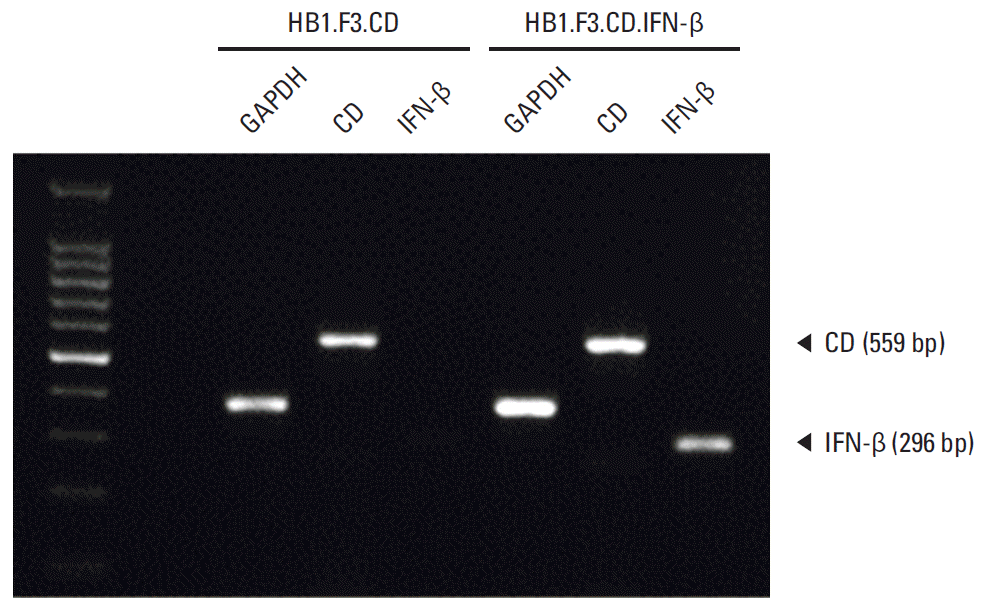
Fig. 2.
In vitro antiproliferative effect of genetically engineered human neural stem cells (hNSCs) with 5-fluorocytosine (5-FC). Since SW-620 cells (4×103 cells/well) were seeded in 96-well plates, after 24 hours, hNSCs were seeded in the same well and treated with 5-FC for 3 days. Cell viability was measured by 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) assay. (A) Cytotoxic effect of various concentrations of 5-FC or 5-fluorouracil (5-FU) on SW-620 cells. (B) Effect of hNSCs with 5-FC (100, 200, 300, 400, and 500 μg/mL) on the SW-620 co-culture system. (C) Microscopic analysis of cells (×200) after 3 days following treatment with hNSCs in the presence of 5-FC (500 μg/mL). Phosphate buffered saline (PBS) treatment was used as a negative control. Data are represented as mean±standard error of the mean. *p < 0.05 vs. control.
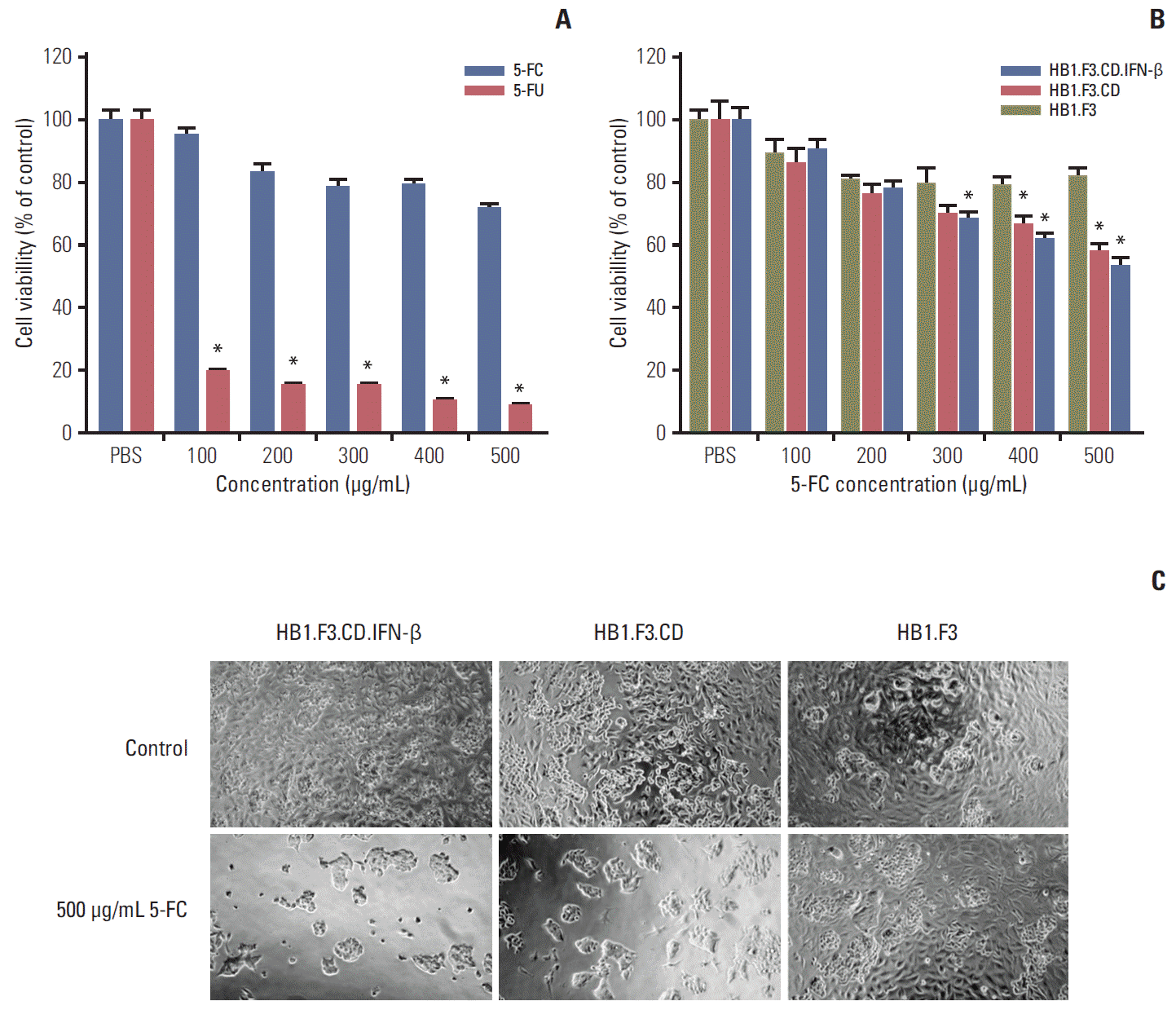
Fig. 3.
Migratory capacity of human neural stem cells (hNSCs) towards SW-620 in vitro. (A) Human fibroblasts (hFB) and SW-620 cells (1×105 cells/well) were seeded on the lower wells of 24-well plates. hNSCs (1×105 cells/well) were stained with CM-Dil and seeded in the fibronectin precoated upper insert chambers. DAPI staining solution was added to lower wells for observation of SW-620 and human fibroblasts. The blue stained cells indicated SW-620 or hFB as a control. Red stained cells indicated migration of hNSCs from the upper insert chamber toward SW-620 or hFB. (B) Migratory ratio of hNSC was measured using a Cell Sense Dimension system (Olympus, Tokyo, Japan). Data are represented as mean±standard error of the mean. *p < 0.05 vs. hFB. (C) Expression of diverse chemoattractant factors was shown in SW-620 cells. SDF-1a, stromal cell-derived factor 1a; uPAR, urokinase receptor; CCR2, C-C chemokine receptor type 2; uPA, urokinase-type plasminogen activator; GAPDH, glyceraldehydes-3-phosphate dehydrogenase.
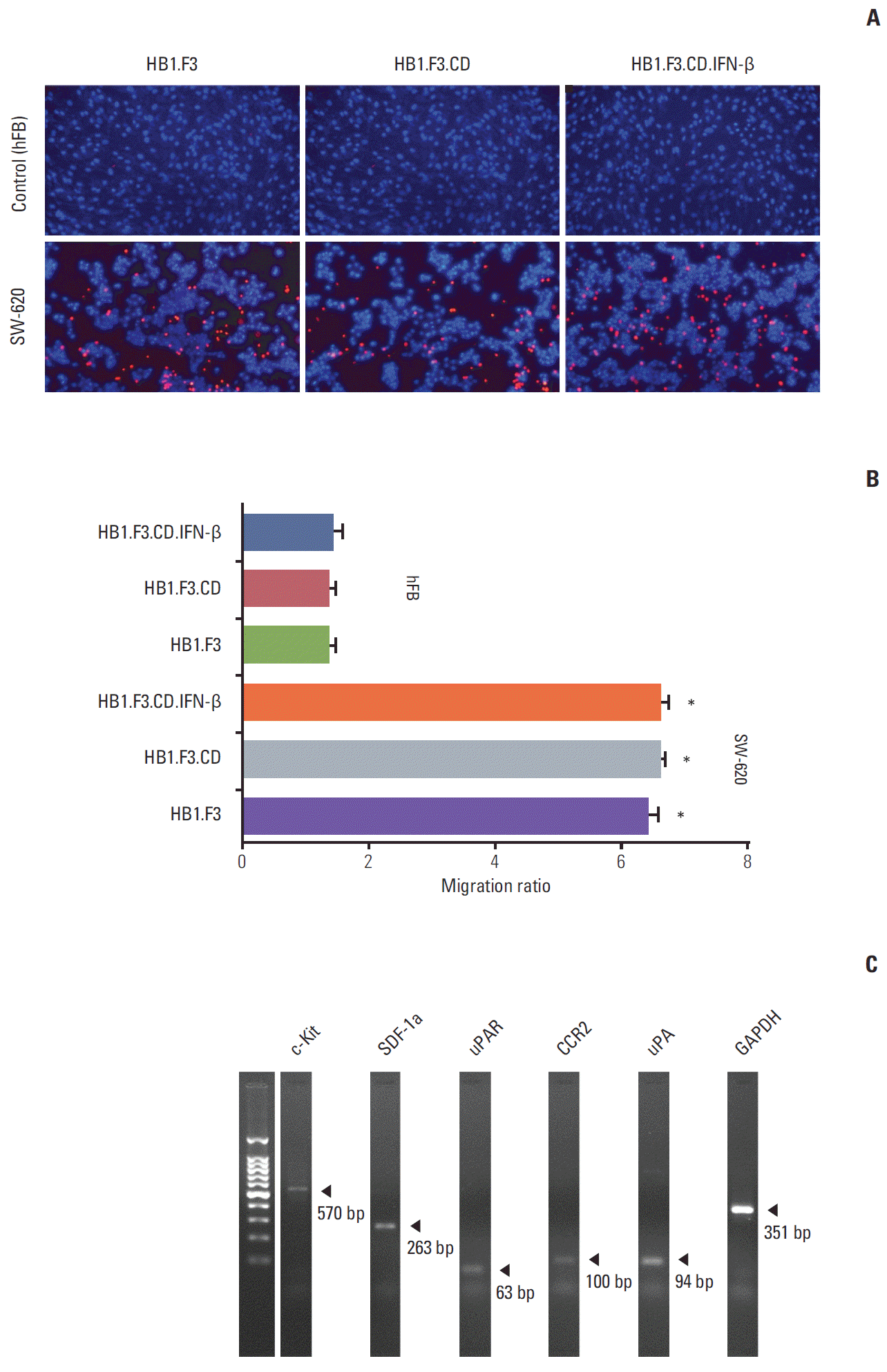
Fig. 4.
Changes in tumor volume following human neural stem cell (hNSC) treatment. A xenograft model was established by implanting SW-620 cells (1×106 cells) in female BALB/c nude mice. (A) Two weeks after SW-620 injection, most tumor masses reached 500 mm3, CM-Dil pre-stained hNSCs (4×106 cells) were injected in a nearby tumor mass. After 48 hours, 5-fluorocytosine (5-FC, 500 mg/kg/day) was administered intraperitoneal every day. (B) Tumor volumes were measured for 21 days and calculated by length×width×height×0.5236 (mm3). A graph showed the changes in tumor volume among treatments with hNSCs in the presence of 5-FC at the termination of the experiment. Data are represented as mean±standard error of the mean. *p < 0.05 vs. phosphate buffered saline (PBS) treatment alone.
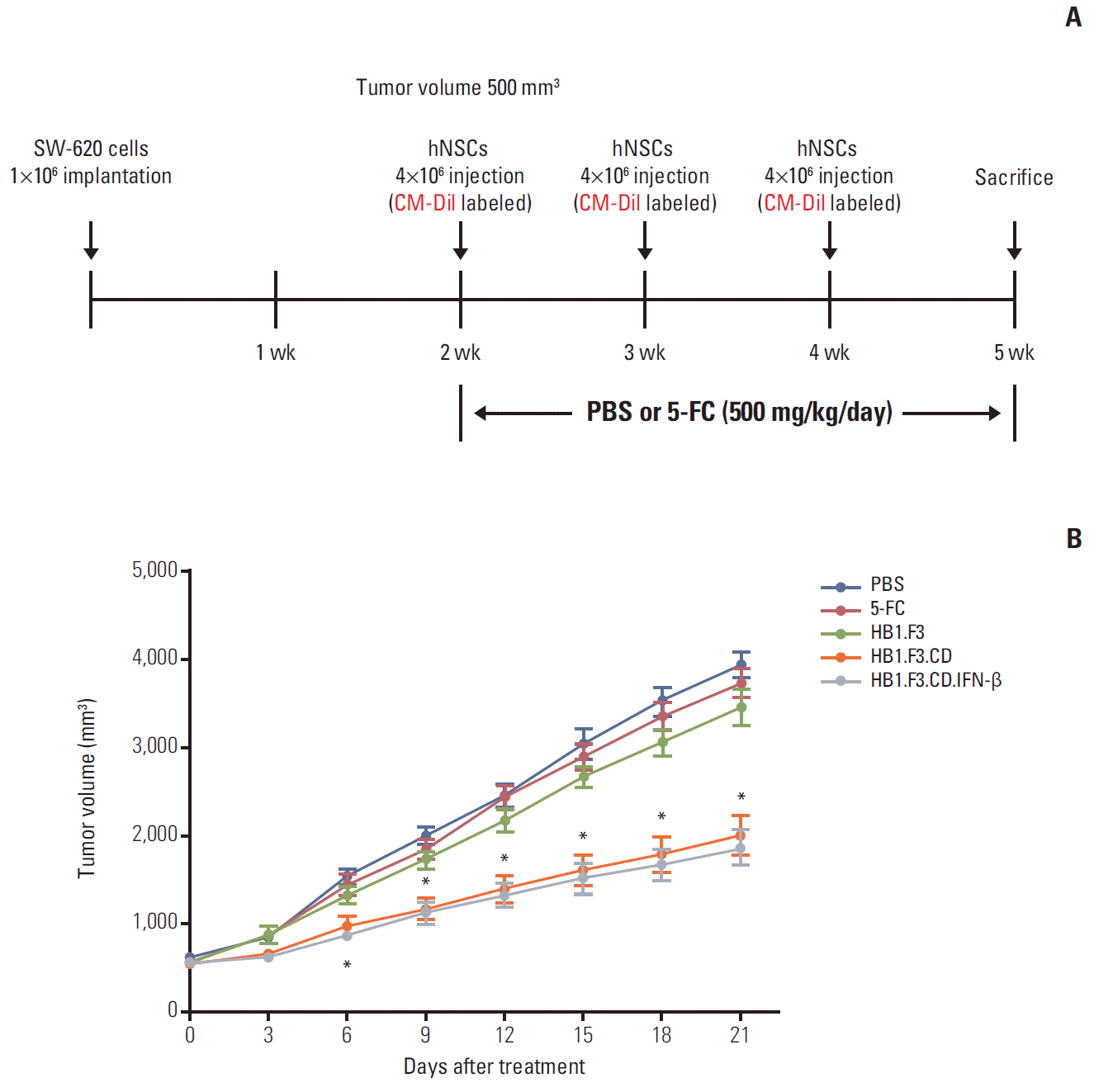
Fig. 5.
Fluorescence analysis of tumor mass in a xenografted mouse model. During the experimental period, human neural stem cells (hNSCs) were injected after pre-labeling with CM-Dil cell tracker. After preparation of tumor sections, DAPI counterstaining was performed. The stained section was observed by fluorescence microscopy. Blue and red indicate DAPI stained nuclei of SW-620 cells and CM-Dil labeled hNSCs, respectively. PBS, phosphate buffered saline.
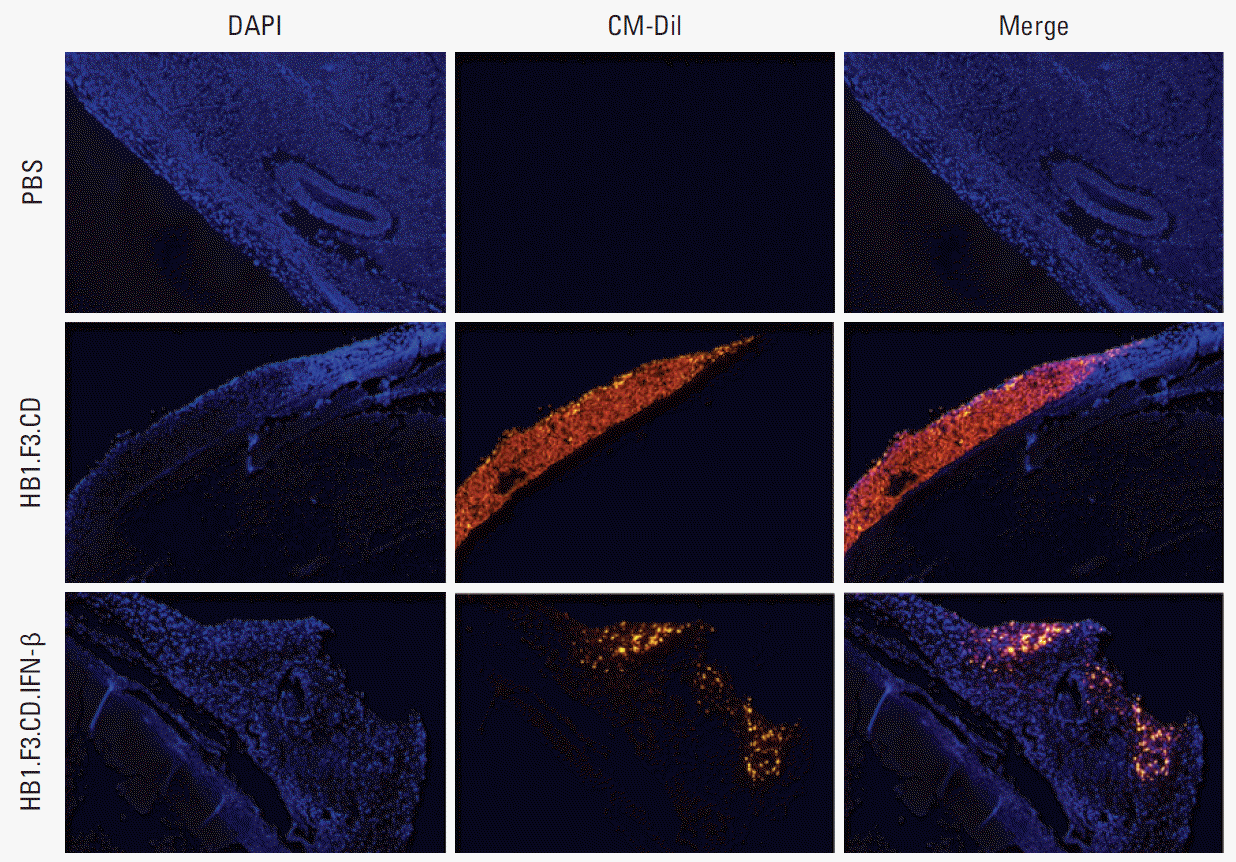
Fig. 6.
Immunohistopathological analysis of proliferating cell nuclear antigen (PCNA) in tumor mass in a xenografted mouse model. Tumor masses were excised from each group of mice treated with human neural stem cells in the presence or absence of a prodrug. The tumor masses were fixed with 10% formalin, cut into 5-mm-thick sections, embedded in paraffin, and sectioned using a microtome (5 μm). (A) Each section was incubated with a primary antibody specific for PCNA. (B) PCNA expression was calculated and shown in the graph. Data are represented as mean±standard error of the mean. *p < 0.05 vs. phosphate buffered saline (PBS) control. 5-FC, 5-fluorocytosine.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download