Introduction
Materials and Methods
1. Patient sample
2. Treatment protocols
3. Follow-up and evaluations
4. Statistical analysis
Results
1. Patient characteristics
2. Survival outcome and progression
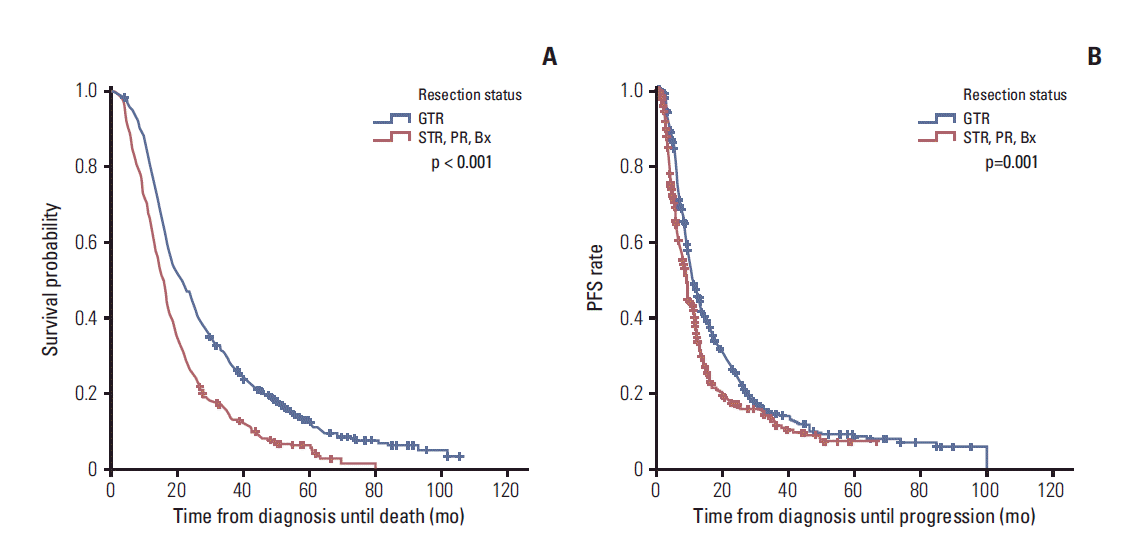 | Fig. 1.Kaplan-Meier curves showing overall survival (OS) (A) and progression-free survival (PFS) (B) according to the extent of the resection. Patients who received gross total resection (GTR) showed a significantly longer OS (21.0 months vs. 15.8 months) and PFS (10.9 months vs. 9.1 months) than those who received subtotal resection (STR), partial resection (PR), or biopsy (Bx) alone. |
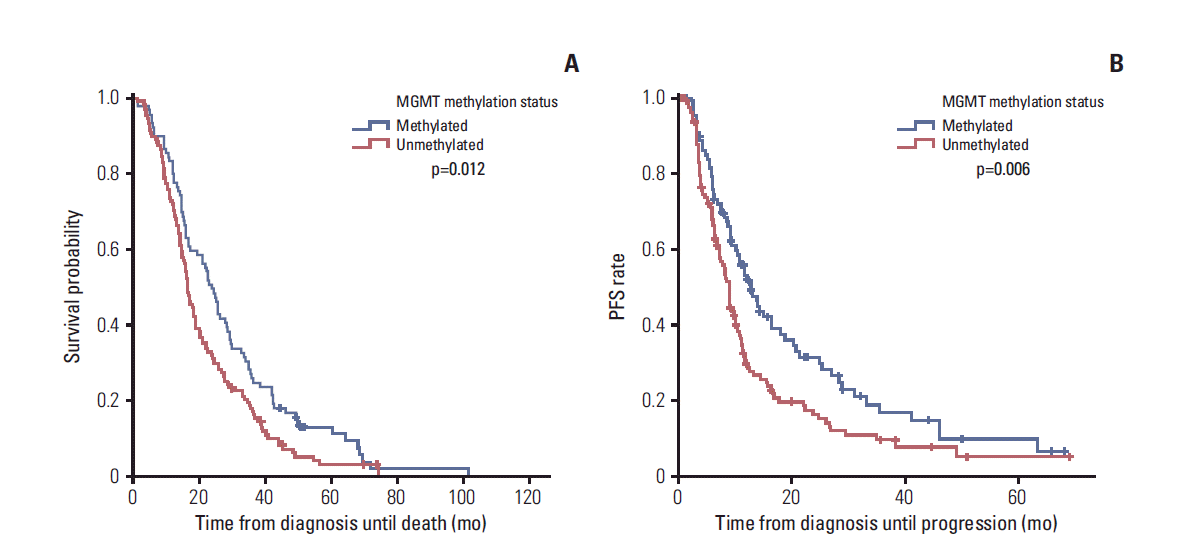 | Fig. 2.Kaplan-Meier curves showing overall survival (OS) (A) and progression-free survival (PFS) (B) according to the O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status. Patients with the methylated MGMT promoter had a longer OS (23.9 months vs. 16.7 months) and longer PFS (13.2 months vs. 9.3 months) than those with the unmethylated MGMT promoter. |
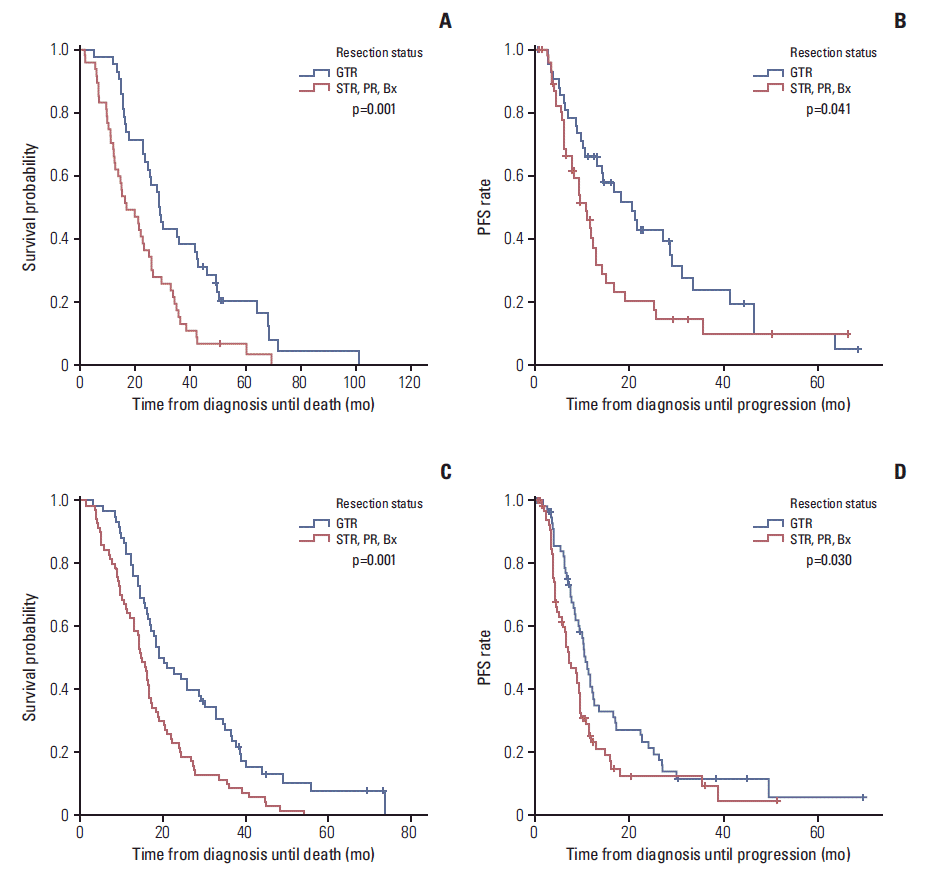 | Fig. 3.Kaplan-Meier curves showing overall survival (OS) (A) and progression-free survival (PFS) (B) according to the extent of the resection in patients with the methylated O6-methylguanine-DNA methyltransferase (MGMT) promoter. Kaplan-Meier curves showing OS (C) and PFS (D) according to the extent of resection in patients with unmethylated MGMT promoter. Patients receiving gross total resection (GTR) demonstrated a significantly longer OS than those receiving subtotal resection (STR), partial resection (PR), or biopsy (Bx) alone in both groups with methylated (28.6 months vs. 16.7 months) and unmethylated MGMT promoter (19.0 months vs. 14.8 months). For OS, patients receiving GTR demonstrated a significantly longer PFS than those receiving STR, PR, or Bx alone in both groups with methylated (20.7 months vs. 11.1 months) and unmethylated MGMT promoter (10.6 months vs. 7.2 months). |
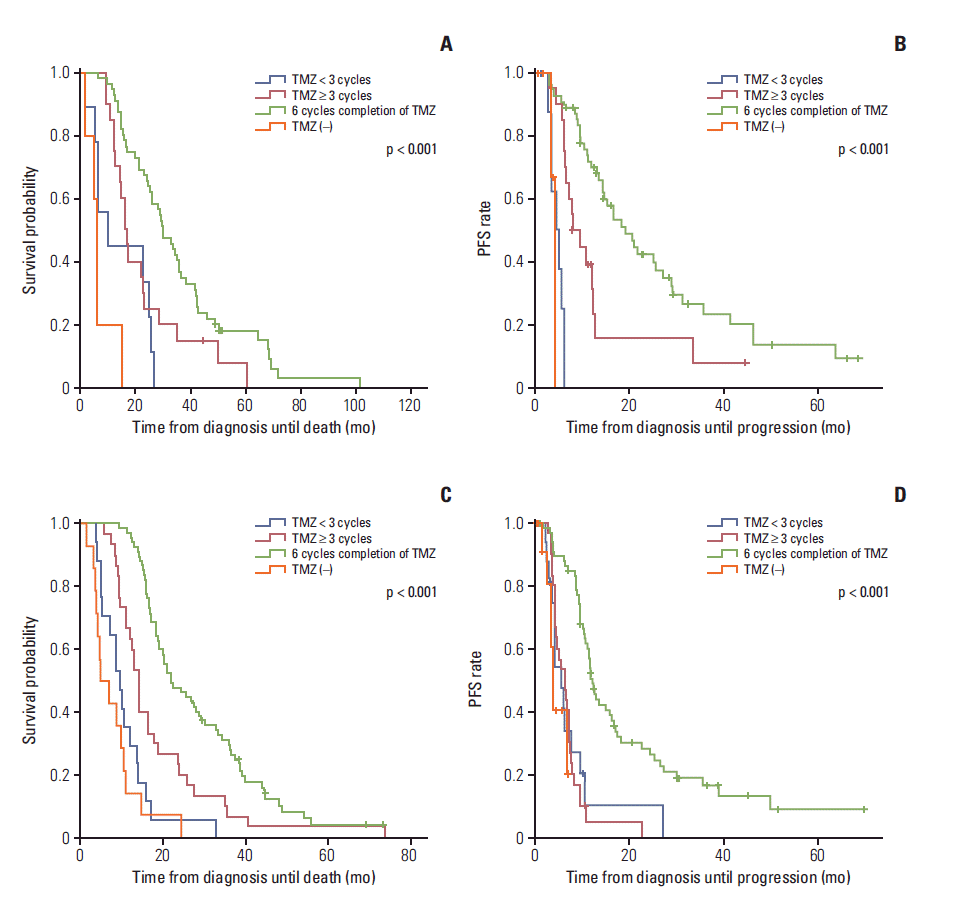 | Fig. 4.Kaplan-Meier curves showing overall survival (OS) (A) and progression-free survival (PFS) (B) according to the completion of six cycles of adjuvant temozolomide (TMZ) in patients with the methylated O6-methylguanine-DNA methyltransferase (MGMT) promoter. Kaplan-Meier curves showing OS (C) and PFS (D) according to completion of six cycles of adjuvant TMZ in patients with the unmethylated MGMT promoter. Patients with the methylated MGMT promoter had a longer OS and longer PFS as the number of adjuvant TMZ cycles approached six compared to those with the unmethylated MGMT promoter. |
Table 1.
3. Treatment-related hematologic toxicity
Discussion
Table 2.
| Characteristics | Stupp et al. (2005) [5,18] | Oike et al. (2013) [10] | Park et al. (2013) [25] | Joo et al. (2012) [24] | Present study |
|---|---|---|---|---|---|
| Patients enrolled | 287 | 45 | 50 | 103 | 750 |
| Age (yr) | 56 (19-70) | 60 (5-79) | 49 (28-70) | 57 (18-77) | 57.5 (20-86) |
| Extent of resection | |||||
| GTR | 113 (39) | 7 (15.6) | 20 (40) | 57 (55) | 388 (51.7) |
| STR | 126 (44) | 34 (75.6) | 30 (60) | 18 (18) | 159 (21.2) |
| PR | 6 (6) | 96 (12.8) | |||
| Biopsy | 48 (17) | 4 (8.8) | 12 (12) | 22 (21) | 107(14.3) |
| Status of MGMT promoter | |||||
| Methylated | 46 (43.4) | 21 (47.7) | 14 (28) | 14 (47) | 89 (41.0) |
| Unmethylated | 60 (56.6) | 23 (52.3) | 36 (72) | 16 (53) | 128 (59.0) |
| Not assessed | 181 | 1 | - | 73 | 533 |
| Six cycles of adjuvant TMZ completed after CCRT | 105 (47) | - | 26 (52) | 54 (52) | 407 (61.7) |
| Cycles of adjuvant TMZ | 3 (0-7) | 7 (0-54) | - | 6 (1-15) | 6 (0-15) |
| Dose of RT (BED, Gy) | 60 (12-62) | 72 (65-72) | 60 | 59.4 (0.8-70.0) | 60 (4.0-84.0) |
| OS (95% Cl, mo) | 14.6 (13.2-16.8) | 15.8 (12.3-19.3) | 23.0 (16.7-29.3) | 18.0 (14.8-21.2) | 17.5 (16.5-18.5) |
| 1-Yr OS (%) | 61.1 | 71.1 | - | 73.7 | 72.1 |
| 2-Yr OS (%) | 26.5 | 27.7 | - | 38.0 | 36.0 |
| 3-Yr OS (%) | - | 21.6 | - | - | 21.0 |
| PFS (95% Cl, mo) | 6.9 (5.8-8.2) | - | 9.0 (5.5-12.5) | 10.0 (8.8-11.2) | 10.1 (9.3-10.9) |
| Grade 3 or 4 hematologic toxicity | 19 (7) | 9 (20.4) | 1 (2) | 8 (8.1) | 115 (15.3) |
Values are presented as number (%) or median (range) unless otherwise indicated. OS, overall survival; PFS, progression-free survival; GTR, gross total resection; STR, subtotal resection; PR, partial resection; MGMT, O6-methylguanine-DNA methyltransferase; TMZ, temozolomide; CCRT, concurrent chemoradiotherapy with TMZ; RT, radiotherapy; BED, biologically effective dose; CI, confidence interval.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download