Abstract
Purpose
A meta-analysis was conducted to examine the question of whether combination regimens are more effective than monotherapy as a second-line chemotherapy in advanced gastric cancer.
Materials and Methods
The MEDLINE and the EMBASE databases and the Cochrane Central Register for Controlled Trials were searched using appropriate keywords. Only randomized controlled trials were eligible.
Results
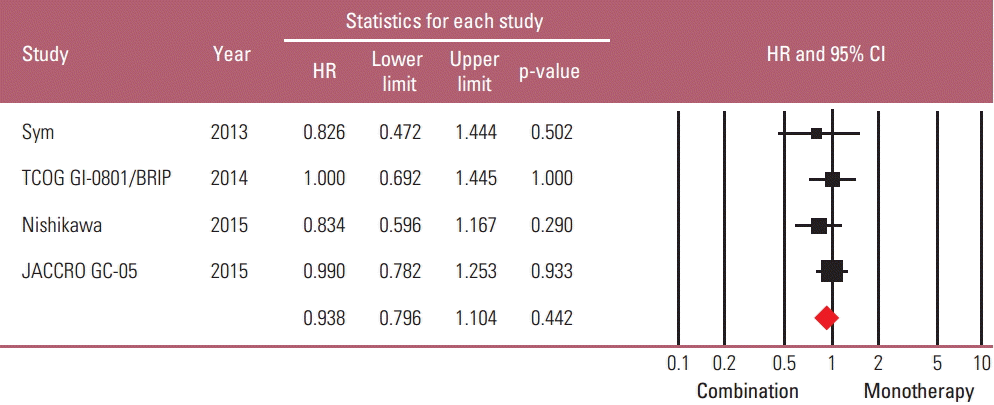
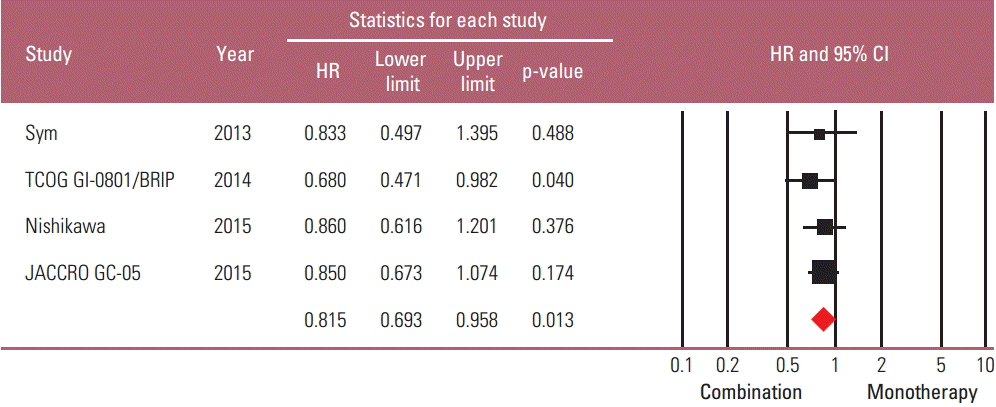
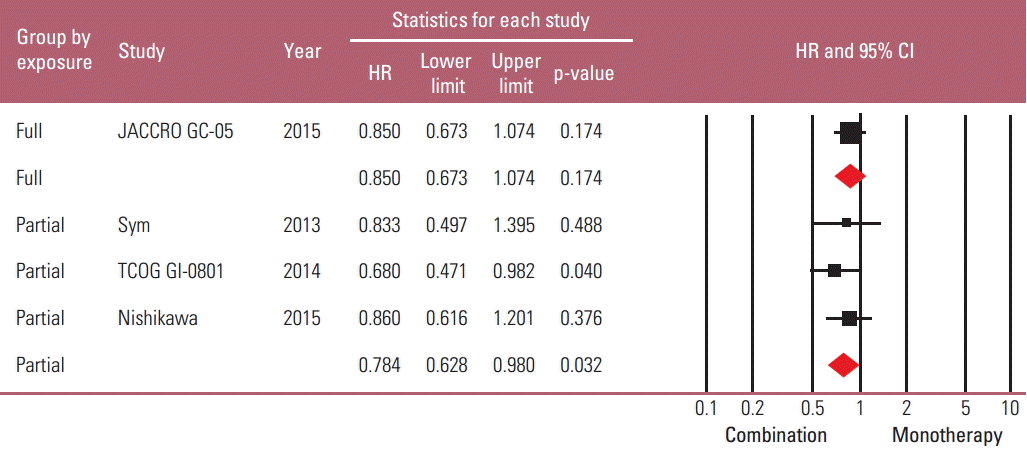
Taxane-based study is rare; thus, four irinotecan-based studies were finally included in the meta-analysis. Out of 661 patients, 331 patients were assigned to combination therapy and 330 to monotherapy. Cisplatin or fluoropyrimidine (S-1 or 5-fluorouracil) was used as a combination partner to irinotecan. The pooled hazard ratio (HR) for overall survival (OS) and for progression-free survival (PFS) was 0.938 (95% confidence interval [CI], 0.796 to 1.104; p=0.442) and 0.815 (95% CI, 0.693 to 0.958; p=0.013). In subgroup analysis according to previous exposure to a partner agent, the PFS benefit of combination was observed only in the partially exposed group (HR, 0.784; 95% CI, 0.628 to 0.980; p=0.032).
Gastric cancer is one of the most common cancer types worldwide. While its incidence and related mortality is declining, its prevalence is still high, accounting for a major cause of cancer death worldwide including East Asian countries [1,2].
The prognosis of metastatic or recurrent gastric cancer is generally poor and only palliative chemotherapy was proven to provide a survival benefit [3]. Various combination regimens of fluoropyrimidine and platinum with/without trastuzumab, according to the human epidermal growth factor receptor 2 status, are commonly used as the front-line chemotherapy [4], and combination regimens were shown to be superior to single-drug regimen in terms of overall survival (OS) [3].
In cases of disease progression after the first-line chemotherapy, second-line chemotherapy also appears to provide a survival benefit. Patient survival was significantly prolonged by either docetaxel or irinotecan monotherapy in comparison with best supportive care [5]. In addition, in comparison of weekly paclitaxel and irinotecan as a second-line regimen, no difference in terms of survival or tolerability was reported between these two regimens [6]. Therefore, taxane or irinotecan monotherapy is currently regarded as two equivalent types of standard second-line chemotherapy. In a recent study, ramucirumab, vascular endothelial growth factor receptor 2 antibody, was shown to provide an additional survival benefit when added to weekly paclitaxel as second-line treatment [7].
However, we still do not know whether combination chemotherapy is better than monotherapy in the second-line setting. In fact, combination chemotherapy is still being used in clinical practice. According to a large retrospective study conducted in a single Korean center, out of 725 patients who received second-line chemotherapy, 218 patients were treated with monotherapy, but 507 patients were treated with combination regimen [8]. Clinical trials comparing combination chemotherapy with monotherapy in this setting have also been conducted.
A meta-analysis of these trials was conducted to examine the question of whether combination regimens are more effective than monotherapy as a second-line therapy in advanced gastric cancer (AGC).
We searched the MEDLINE and the EMBASE databases and the Cochrane Central Register for Controlled Trials up to 15 July 2015 using guidelines developed for reporting a systematic review [9,10]. The following keywords were used in the search: “gastric or gastro-esophageal or stomach,” “cancer or tumor or malignancy or carcinoma,” “chemotherapy,” and “second-line or salvage.” The titles and abstracts were checked to exclude clearly unrelated articles. All searches were performed independently by the two authors (Y.-H.C and S.Y.Y.).
Clinical trials that met the following criteria were included in the meta-analysis: (1) prospective randomized trials; (2) trials comparing a single-drug regimen with a combination regimen as the second-line chemotherapy in AGC patients; (3) trials with available survival data, including progression-free survival (PFS) and OS and hazard ratio (HR) thereby estimated using the 95% confidence interval (CI).
Data were extracted from the included studies by two authors (Y.-H.C. and S.Y.Y.). The name of the study or the first author, the year of publication, the study design, the study location, sample size, randomization methods, chemotherapy regimens, follow-up, and survival data were extracted and reviewed. Discrepancies in data extraction were jointly reviewed until a consensus was reached. Study quality was evaluated independently by two authors (Y.-H.C. and S.-N.K.) using the Cochrane Risks of Bias assessment [11].
The primary outcomes of this meta-analysis were pooled HR for OS and PFS for the combination regimen versus monotherapy. For pooled HR analysis, a fixed effects model was used in the absence of significant heterogeneity across the trials. Study heterogeneity was examined by I2, which measures the percentage of the total variation across studies and a value greater than 50% defines substantial heterogeneity [12,13]. When heterogeneity was observed, a random effects model was used to estimate the combined HR. A sensitivity analysis was performed to examine the robustness of conclusions by eliminating each study in the meta-analysis one at a time to determine its effect on the pooled HR. Publication bias was evaluated using the Begg-Mazumdar rank correlation test [14], and a funnel plot was constructed for assessment of this bias [15]. Statistical tests were performed using Comprehensive Meta-Analysis ver. 2.0 (Biostat, Englewood, NJ), and p < 0.05 was considered significant.
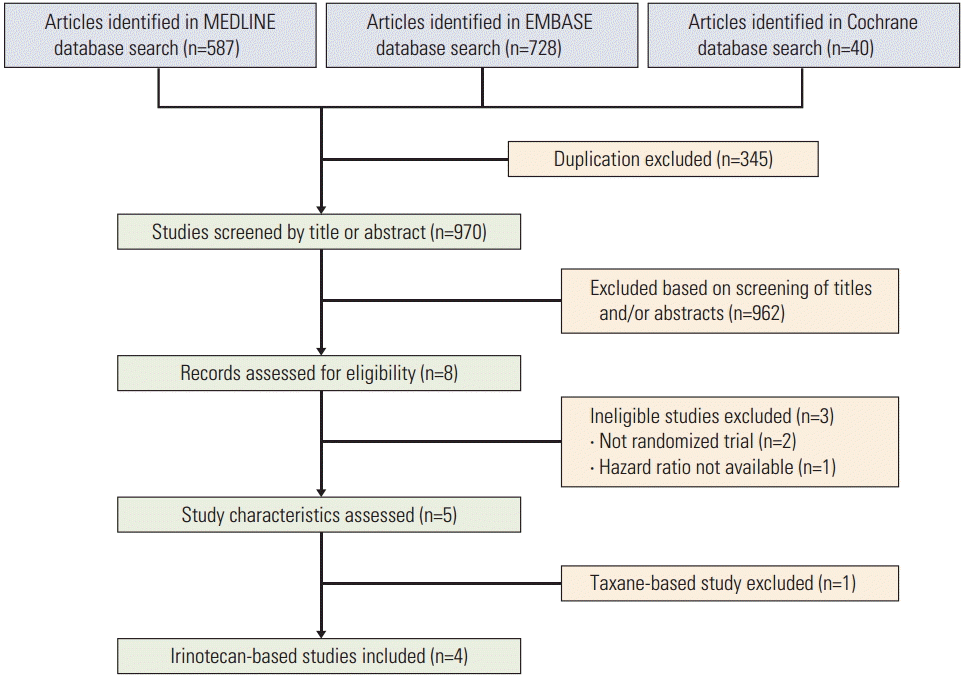
Initially 1,355 articles were found from three databases using the pre-defined keywords. After excluding 345 duplications, 970 articles were identified, which were then screened by the title or the abstract. Eight articles or abstracts were considered appropriate and were assessed for eligibility; two studies were not randomized trials and one study did not report the HR for survival. Out of the five remaining studies, four studies compared irinotecan monotherapy with combination and the other study used either irinotecan or paclitaxel as monotherapy. Due to the rarity of taxane-based study, we decided to focus only on irinotecan-based studies. Finally, four irinotecan-based studies were included in the meta-analysis (Fig. 1).
All studies were published relatively recently (Table 1) [16-19]. Two studies evaluated cisplatin as the combination partner to irinotecan and two other studies evaluated fluoropyrimidine (S-1 or 5-fluorouracil [5-FU]). Except one study, variable proportions (56%-100%) of patients in the combination therapy group were previously exposed to the partner agents. In particular, one Japanese study evaluated S-1 combination in all S-1–refractory patients [19].
While three studies reported no difference in terms of the OS or PFS between two groups, the TCOG GI-0801/BIRIP study reported better PFS, but similar OS in the combination therapy compared with the monotherapy [17].
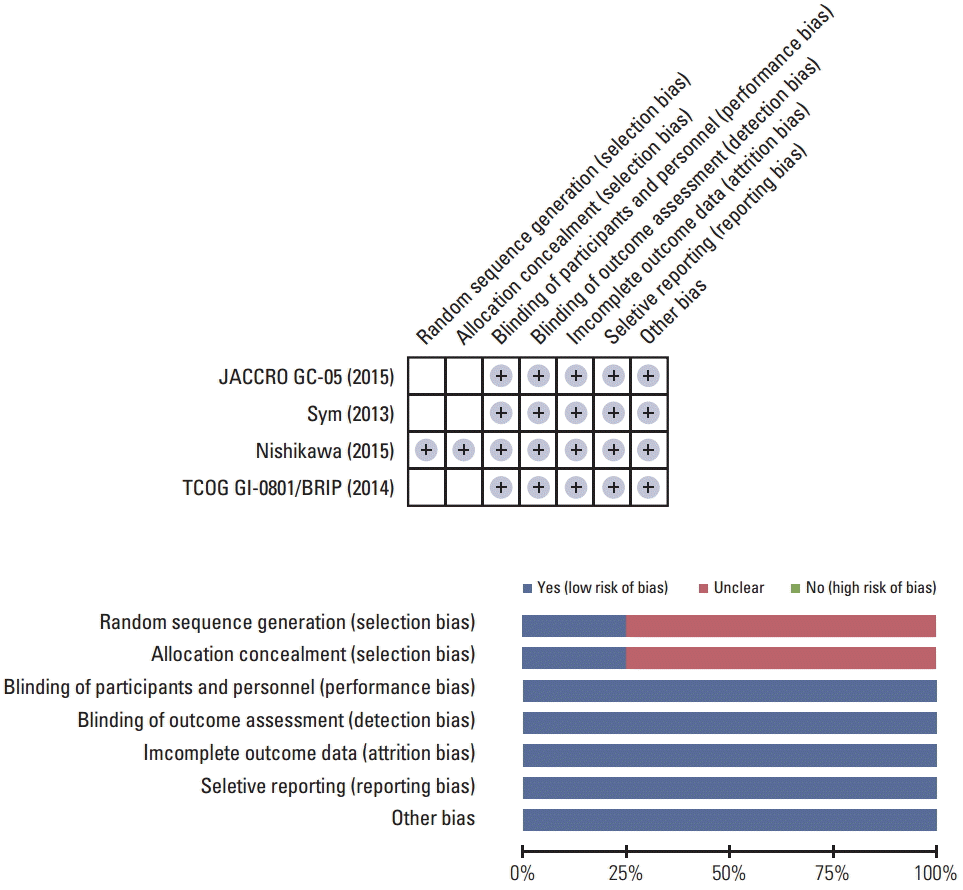
According to the Cochrane Risk of Bias assessment, three trials had “unclear” risk of selection biases because they did not specify randomization methods. Otherwise, there was no additional risk of bias in all trials (Fig. 2).
A total of 661 patients were included in the meta-analysis; 331 patients were assigned to combination treatment and 330 to monotherapy. The pooled HR for OS and PFS for the combination versus irinotecan monotherapy was 0.938 (95% CI, 0.796 to 1.104; p=0.442) and 0.815 (95% CI, 0.693 to 0.958; p=0.013) (Figs. 3 and 4). Thus, a significant risk reduction of progression was observed with combination therapy, although survival was not increased. The fixed model was applied since heterogeneity was not detected across the studies of OS (p=0.911 and I2 =0.000) and PFS (p=0.688 and I2 =0.000). In the sensitivity analysis, which was performed to examine the robustness of data, no individual study had a significant effect on the pooled HR for both OS and PFS. The funnel plot drawn for the assessment of publication bias showed symmetricity of the studies, and the Begg-Mazumdar rank correlation test also showed no evidence of publication bias in this meta-analysis.
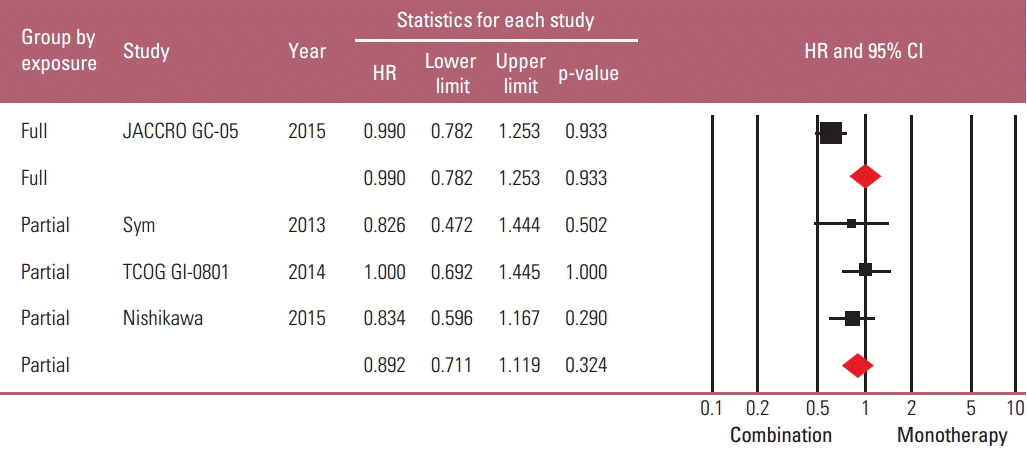
Because it seemed possible that the previous exposure to the partner drug could affect the therapeutic efficacy of combination regimens, a subgroup analysis was performed based on the degree of previous exposure to the chemoagents used in combination. The “full exposure” group (one Japanese study with S-1 combination in all S-1–exposed patients) was compared with the “partial exposure” group (remaining three studies in which up to 56% of patients were previously exposed to the partner drugs). The risk reduction of progression by combination regimen was observed only in the “partial exposure” group (HR, 0.784; 95% CI, 0.628 to 0.980; p=0.032), not in the “full exposure” group (HR, 0.85; 95% CI, 0.673 to 1.074; p=0.174) (Figs. 5 and 6).
In this meta-analysis, irinotecan-based combination chemotherapy was associated with better PFS, i.e., approximately 18% risk reduction of progression over irinotecan monotherapy. However, this prolonged PFS did not translate to gain of OS, which is not an uncommon observation in cancer chemotherapy trials. The reason for this is not obvious; however, some potential explanations could be offered as follows. First, because the treatment beyond progression was not controlled in these trials, there might have been an imbalance of post-progression therapy between the two arms. Second, due to small sample size, it is also possible that the meta-analysis was underpowered to show a survival difference, despite a PFS benefit. A biological speculation was also proposed that a combination therapy could delay progression for a time but lead to a more aggressive phenotype after treatment, thus offsetting the earlier delay in progression [20].
Some patients in the combination arm were treated with previously exposed drugs. Re-administration of a previously exposed drug is not uncommon in cancer chemotherapy. In the case of metastatic colorectal cancer or non-small cell lung cancer, 5-FU/leucovorin or cisplatin is often maintained in the second-line treatment. However, according to the subgroup analysis in this meta-analysis, the degree of previous exposure appears to affect patients’ outcome, i.e., only the “partial exposure” group showed a significant risk reduction of progression. Thus, in order to enhance the therapeutic efficacy of second-line therapy, a combination with a new agent might be more desirable. However, due to the limitation of available active agents, use of non-cross-resistant derivatives may be a more realistic alternative. A small phase II trial comparing weekly docetaxel plus oxaliplatin with docetaxel alone in 52 metastatic gastric cancer patients previously treated with cisplatin based regimen [21] reported a significant prolongation of PFS (median PFS, 4.93 months vs. 1.97 months; p=0.007) and an equivalent OS (median OS, 8.1 months vs. 7.2 months; p=0.353) in the doublet arm.
Oxaliplatin, a platinum derivative, showed substantial activity in cisplatin-refractory metastatic gastric cancer [22]. Newer oral fluoropyrimidines, such as capecitabine and S-1, are currently available, although their cross-resistance with intravenous 5-FU has not been determined. Further studies may be warranted.
A few limitations of this meta-analysis should be noted.
First, the number of patients was relatively small. Except one large Japanese trial, which tested S-1 combination in all S-1 exposed patients, the three remaining studies were even smaller. The small sample size might not have been sufficient to reflect general gastric cancer patients in the second-line setting.
Second, only irinotecan-based studies were included in the meta-analysis. Thus, we still may not be able to make a conclusion regarding taxane-containing combinations in this setting.
Third, details regarding relevant clinical and pathological factors, which might have influenced the patients’ outcome, were not available and therefore could not be analyzed. For example, in the above mentioned study by Nishikawa et al. [18], irinotecan/cisplatin combination was significantly more effective for intestinal-type AGC, but not for diffuse type.
References
1. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013; 45:1–14.

2. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010; 127:2893–917.

3. Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010; (3):CD004064.

4. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–97.

5. Kim HS, Kim HJ, Kim SY, Kim TY, Lee KW, Baek SK, et al. Second-line chemotherapy versus supportive cancer treatment in advanced gastric cancer: a meta-analysis. Ann Oncol. 2013; 24:2850–4.

6. Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013; 31:4438–44.

7. Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014; 15:1224–35.

8. Ji SH, Lim DH, Yi SY, Kim HS, Jun HJ, Kim KH, et al. A retrospective analysis of second-line chemotherapy in patients with advanced gastric cancer. BMC Cancer. 2009; 9:110.

9. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000; 283:2008–12.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097.

11. Higgins JP, Green S. Cochrane handbook for systematic review of interventions [Internet]. London: The Cochrane Collaboration;2011. [cited 2016 Apr 1]. Available from: http://handbook.cochrane.org/chapter_8/8_assessing_risk_of_bias_in_included_studies.htm.
12. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–58.

13. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–60.

14. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50:1088–101.

15. Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001; 323:101–5.

16. Sym SJ, Hong J, Park J, Cho EK, Lee JH, Park YH, et al. A randomized phase II study of biweekly irinotecan monotherapy or a combination of irinotecan plus 5-fluorouracil/leucovorin (mFOLFIRI) in patients with metastatic gastric adenocarcinoma refractory to or progressive after first-line chemotherapy. Cancer Chemother Pharmacol. 2013; 71:481–8.

17. Higuchi K, Tanabe S, Shimada K, Hosaka H, Sasaki E, Nakayama N, et al. Biweekly irinotecan plus cisplatin versus irinotecan alone as second-line treatment for advanced gastric cancer: a randomised phase III trial (TCOG GI-0801/BIRIP trial). Eur J Cancer. 2014; 50:1437–45.

18. Nishikawa K, Fujitani K, Inagaki H, Akamaru Y, Tokunaga S, Takagi M, et al. Randomised phase III trial of second-line irinotecan plus cisplatin versus irinotecan alone in patients with advanced gastric cancer refractory to S-1 monotherapy: TRICS trial. Eur J Cancer. 2015; 51:808–16.

19. Tanabe K, Fujii M, Nishikawa K, Kunisaki C, Tsuji A, Matsuhashi N, et al. Phase II/III study of second-line chemotherapy comparing irinotecan-alone with S-1 plus irinotecan in advanced gastric cancer refractory to first-line treatment with S-1 (JACCRO GC-05). Ann Oncol. 2015; 26:1916–22.

20. Booth CM, Eisenhauer EA. Progression-free survival: meaningful or simply measurable? J Clin Oncol. 2012; 30:1030–3.

21. Kim JY, Ryoo HM, Bae SH, Kang BW, Chae YS, Yoon S, et al. Multi-center randomized phase II study of weekly docetaxel versus weekly docetaxel-plus-oxaliplatin as a second-line chemotherapy for patients with advanced gastric cancer. Anticancer Res. 2015; 35:3531–6.
Table 1.
Characteristics of the studies included in the meta-analysis
| Study | Year | Regimen | No. | PFS (mo) | HR_PFS | OS (mo) | HR_OS | Comment |
|---|---|---|---|---|---|---|---|---|
| Sym et al. [16] | 2013 | FOLFIRI | 30 | 3.0 | 0.83 | 6.7 | 0.83 | Previous 5-FU (46%) |
| Irinotecan | 29 | 2.2 | p=0.481 | 5.8 | p=0.514 | |||
| TCOG GI-0801 [17] | 2014 | Irinotecan+cisplatin | 64 | 3.8 | 0.68 | 10.7 | 1.00 | Previous cisplatin (56%) |
| Irinotecan | 66 | 2.8 | p=0.0398 | 10.1 | p=0.982 | |||
| Nishikawa et al. [18] | 2015 | Irinotecan+cisplatin | 84 | 4.6 | 0.86 | 13.9 | 0.834 | No previous platinum |
| Irinotecan | 84 | 4.1 | p=0.376 | 12.7 | p=0.288 | |||
| JACCRO GC-05 [19] | 2015 | Irinotecan+S-1 | 153 | 3.8 | 0.85 | 8.8 | 0.99 | All S-1 refractory |
| Irinotecan | 151 | 3.4 | p=0.16 | 9.5 | p=0.92 |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download