Abstract
Purpose
The purpose of this study is to evaluate the effect of diabetes mellitus (DM) and preoperative glycemic control on prognosis in Korean patients with upper tract urothelial carcinoma (UTUC) who underwent radical nephroureterectomy (RNU).
Materials and Methods
A total of 566 patients who underwent RNU at six institutions between 2004 and 2014 were reviewed retrospectively. Kaplan-Meier and Cox regression analyses were performed to assess the association between DM, preoperative glycemic control, and recurrence-free, cancer-specific, and overall survival.
Results
The median follow-up period was 33.8 months (interquartile range, 41.4 months). A total of 135 patients (23.8%) had DM and 67 patients (11.8%) had poor preoperative glycemic control. Patients with poor preoperative glycemic control had significantly shorter median recurrence-free, cancer-specific, and overall survival than patients with good preoperative glycemic control and non-diabetics (all, p=0.001). In multivariable Cox regression analysis, DM with poor preoperative glycemic control showed association with worse recurrence-free survival (hazard ratio [HR], 2.26; 95% confidence interval [CI], 1.31 to 3.90; p=0.003), cancer-specific survival (HR, 2.96; 95% CI, 1.80 to 4.87; p=0.001), and overall survival (HR, 2.13; 95% CI, 1.40 to 3.22; p=0.001).
Conclusion
Diabetic UTUC patients with poor preoperative glycemic control had significantly worse oncologic outcomes than diabetic UTUC patients with good preoperative glycemic control and non-diabetics. Further investigation is needed to elucidate the exact mechanism underlying the impact of glycemic control on UTUC treatment outcome.
The association between diabetes mellitus (DM) and cancer has recently received significant attention due to increases in the prevalence of both diseases. DM is not a single disease, but a group of metabolic disorders characterized by a series of potential confounding factors (obesity, varying levels of metabolic control, profiles of anti‑diabetic treatment and possible chronic complications or comorbidities) that may influence the association between diabetes and cancer [1]. Therefore, the characteristics of cancer and the metabolic abnormalities of their host may influence cancer cell survival, proliferation, and spread.
Upper tract urothelial carcinoma (UTUC), histologically similar to bladder tumor, is less common than bladder cancer [2]. From this perspective, there is little clinical evidence of oncological outcomes and prognostic factors in UTUC after radical nephroureterectomy (RNU), and most well-known prognostic factors are related to tumor factors such as stage, grade, and tumor multifocality. Therefore, the preoperative prognostic factors related to UTUC patients should be identified.
Several studies have demonstrated that patients with DM have greater cancer mortality compared with non DM patients [3,4], and published studies reporting evidence linking DM and bladder cancer showed that DM has a negative effect on bladder cancer prognosis [5,6]. However, to the best of our knowledge, there is a lack of data regarding the prognostic significance of preoperative glycemic control in surgically treated patients with UTUC who have DM.
Therefore, in the current study, we examined the impact of DM and glycemic control on the prognosis of UTUC after RNU.
This study was approved by the Institutional Review Boards of all participating centers. Data from 597 UTUC patients who underwent RNU between 2004 and 2014 were collected from six tertiary medical centers in Korea. Patients with a previous history of bladder cancer, regional lymph node metastasis or distant metastasis (the lymph node status was only purely based on preoperative radiologic findings), or received preoperative chemotherapy were excluded. Patients who had DM (all type2 DM), but in whom the preoperative hemoglobin A1c (HbA1c) level was not checked, were also excluded. Finally, 566 patients were reviewed retrospectively. The patient demographics, perioperative data, pathologic findings, and clinical outcomes, including survival data, were collected retrospectively using a prespecified template for consistent data collection for an electronic medical record review. HbA1c was measured within 6 months preoperatively. Preoperative radiological evaluations included abdominal computed tomography and chest X-ray or computed tomography and (when clinically indicated) positron emission tomography or bone scan. There were no limitations to the surgical approaches or modalities used. For the analysis, the patients were divided into three groups: patients without a history of DM, patients with well controlled DM (HbA1c < 7), and patients with poorly controlled DM (HbA1c ≥ 7). No information regarding the age at diagnosis of DM was collected.
All patients were followed similarly every 3-4 months in the first year after RNU, every 6 months from the second through the fifth year, and annually thereafter. At each follow-up, the patient’s symptoms, history, performance status, and physical examination were evaluated by the physicians, and blood samples for serum chemistry and hematological testing were obtained. Local recurrence and distant metastasis were examined by a chest radiograph, computed tomography, positron emission tomography, or bone scans when clinically indicated. The survival data, including recurrence-free survival (RFS), cancer-specific survival (CSS), and overall survival (OS), were defined from the date of surgery to the date of recurrence, death from UTUC and death from any cause, or were censored at the date of the last follow-up. Recurrence was defined as a new soft-tissue mass > 10 mm that was previously undetected on a computed tomography scan in the operative field, regional lymph nodes, and/or distant organs. Recurrence of bladder cancer was not considered as disease recurrence [7]. A biopsy for tissue confirmation was not routinely performed.
All surgical specimens were processed according to the standard pathological procedures and were reviewed by uro-pathologists. Tumors were staged according to the American Joint Committee on Cancer seventh edition TNM staging system. The tumor grade was assessed according to the 1998 World Health Organization/International Society of Urologic Pathology consensus classification.
Demographic, clinical and pathological data were compared using the Kruskal-Wallis test for continuous variables and the chi-square test for categorical variables among the three groups. Survival analyses were performed using the Kaplan-Meier method with the log rank test. Cox proportional hazard regression analysis was used for identification of independent prognostic factors for each dependent variable. All p-values were two-sided and p < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS ver. 21.0 (IBM Inc., Armonk, NY) and MedCalc for Windows ver. 12.5 (MedCalc Software, Ostend, Belgium).
The median age of enrolled patients was 70.0 years (interquartile range [IQR], 13 years), with a median follow-up period of 33.8 months (IQR, 41.4 months). The clinicopathological characteristics were similar among the no-DM group (n=431), well controlled DM group (n=68), and poorly controlled DM group (n=67) except for recurrence rate, cancer death rate, and rate of death from any cause. The recurrence rate, cancer death rate, and rate of death from any cause were high in the poorly controlled DM group compared to the non-DM and well controlled DM groups (p < 0.05) (Table 1).
The median time to recurrence was 17.7 months (IQR, 28.5 months); 92 patients (16.3%) had disease recurrence after RNU. Of these, 21 patients (3.7%) had local recurrence and 71 patients (12.5%) experienced distant metastasis. The RFS at 3 and 5 years was 77.5% and 72%, respectively. Compared to the non-DM and well controlled DM patients, poorly controlled DM patients showed significantly shorter RFS (no DM vs. HbA1c ≥ 7, p=0.011; HbA1c < 7 vs. HbA1c ≥ 7, p=0.001) (Fig. 1A). However, no difference was observed between non-DM patients and well controlled DM patients (p=0.05) (Fig. 1A). In univariable and multivariable Cox regression analyses, poorly controlled DM was associated with increased risk of disease recurrence (univariable: hazard ratio [HR], 1.96; 95% confidence interval [CI], 1.15 to 3.34; p=0.013; multivariable: HR, 2.26; 95% CI, 1.31 to 3.90; p=0.003) (Tables 2 and 3).
During follow-up, 82 patients (14.4%) died from UTUC. The median time to cancer-specific death was 30.4 months (IQR, 39.3 months). The CSS at 3 and 5 years was 85.5% and 76%, respectively. Compared to the non-DM and well controlled DM patients, poorly controlled DM patients showed significantly shorter CSS (no DM vs. HbA1c ≥ 7, p=0.001; HbA1c < 7 vs. HbA1c ≥ 7, p=0.001) (Fig. 1B), and no significant difference was observed between non-DM and well controlled DM patients (p=0.418) (Fig. 1B). In univariable and multivariable Cox regression analyses, poorly controlled DM was associated with increased risk of cancer-specific mortality (univariable: HR, 2.93; 95% CI, 1.79 to 4.78; p=0.001; multivariable: HR, 2.96; 95% CI, 1.80 to 4.87; p=0.001) (Tables 2 and 3).
With respect to overall mortality, the median time to death from any cause was 30.0 months (IQR, 39.0 months). The OS at 3 and 5 years was 85.5% and 76.7%, respectively. Similar to RFS and CSS, poorly controlled DM patients had significantly shorter OS compared to non-DM and well controlled DM patients (no DM vs. HbA1c < 7, p=0.075; no DM vs. HbA1c ≥ 7, p=0.001; HbA1c < 7 vs. HbA1c ≥ 7, p=0.001) (Fig. 1C). In univariable and multivariable Cox regression analyses, poorly controlled DM was associated with increased risk of overall mortality (univariable: HR, 2.10; 95% CI, 1.41 to 3.12; p=0.002; multivariable: HR, 2.13; 95% CI, 1.40 to 3.22; p=0.001) (Tables 2 and 3). Of note, well controlled DM showed borderline significance in univariable analysis for OS, but lost its significance in multivariable analysis.
A previous study reported that preexisting DM increases the risk of several cancers, including cancer of the breast, colorectum, endometrium, liver, and pancreas [8]. In American Cancer Society Cancer Prevention Study II, adults with DM were at increased risk for cancer related mortality [9]. Other studies have reported a possible association of DM with mortality from cancer of the liver, pancreas, colorectum, lung, and breast [10]. Similarly, diabetes was associated with a statistically significant 1.3- to 2.5-fold increased risk of bladder cancer in previous cohort studies [9,11,12], and studies on cancer mortality have found that bladder cancer patients with diabetes have greater cancer mortality compared with their nondiabetic counterparts [4,9].
UTUC is relatively rare and shares many characteristics with urothelial cancer of the bladder, therefore most decision making regarding UTUC is extrapolated from evidence in bladder cancer [2]. However, there are anatomical, biological, and practical differences between bladder cancer and UTUC. Compared with other malignancies, fewer studies on the potential impact of DM on CSS in patients with UTUC have been reported in the literature. Rieken et al. [7] reported that diabetic patients with UTUC who did not use metformin were at significantly higher risk of disease recurrence and cancer-specific death compared to nondiabetic patients and diabetic patients with UTUC who used metformin. However, there was no information on the state of glucose control and UTUC. Hwang et al. [13] reported that DM was an independent risk factor for RFS in UTUC, but they did not report on the relationship between glycemic control status and long-term prognosis such as CSS and OS. In the current study, we found that diabetic UTUC patients with poor glycemic control showed shorter median RFS, CSS, and OS compared with diabetic UTUC patients with good glycemic control and non-diabetic patients. There was no significant difference between non-DM and well controlled DM patients. There was no significant difference between non-DM and well controlled DM patients. In our study, we used the HbA1c level, which is a more informative measure compared with a simple DM history or a single serum glucose test. The HbA1c level reflects the patient’s recent glycemic control status. Our results showed that glucose regulation status using HbA1c was a clinically significant prognostic factor for predicting the survival of patients with UTUC.
Previous studies reported association of poor glycemic control according to the HbA1c level with worse outcomes in other cancers [14,15]. Lee et al. [15] reported that poor glycemic control was related to disease progression in renal cell carcinoma and proposed that stricter glycemic control would contribute to improved outcomes. Siddiqui et al. [14] also reported an association of elevated HbA1c with aggressive clinical behavior in patients with colorectal cancer. An informative measure such as HbA1c which can reflect the glycemic control status rather than a simple DM history or a single glucose test is warranted in patients with UTUC. In addition, our study suggests that even diabetic patients could have long-term survival comparable with non DM patients through strict glucose control.
The mechanism by which DM contributes to cancer mortality has not been fully elucidated, however plausible explanations have been suggested to explain the relationship between DM and cancer [16,17]. Hyperglycemia can provide more glucose to tumor cells, and hyperinsulinemia elicited by hyperglycemia could lead to activation of insulin/insulinlike growth factor 1, which can influence cancer progression [18]. In addition, hyperglycemia activates various signaling pathways that cooperate to control cancer cell behavior, including proliferation, migration, invasion, and recurrence [19]. The biological mechanism underlying the relationship between DM and its potential promoting effect on urothelial cells is under investigation. In an in vitro experiment, urothelial proliferation was promoted by high-dose insulin [20]. Expression of IGF-receptor I, which can promote cell growth and antiapoptosis, has been reported in invasive urothelial carcinoma of the bladder [21]. In addition, increased advanced glycosylated end products due to poor glycemic control may lead to structural changes such as reduced expression of the subtype E-cadherin, which has been associated with poor oncologic outcome in patients with bladder cancer [5,22], and chronic inflammation and often accompanying obesity may lead to the release of cytokines which can enhance cancer growth [23]. Further research is warranted for a better understanding of the effect of DM on the development and progression of cancer.
This study also included other prognostic factors in UTUC in addition to glucose control status. The most well-established prognostic factors, including tumor stage, grade, and lymphovascular invasion, were also independent prognostic factors in our study [24]. In addition, adjuvant chemotherapy was found to be an adverse prognostic factor for CSS and OS but not with RFS in our multivariate analysis. The reason for this finding may be that because the adjuvant chemotherapy was administered in advanced disease (pathologic stage T3 or T4), it might affect the poor CSS and OS. Indeed, adjuvant chemotherapy in UTUC is still a controversial issue. A recent meta-analysis demonstrated that OS and disease-free survival benefit is only obtained with cisplatin-based combination chemotherapy (CBCC) but not with non-cisplatin–based regimens [25]. Another study reported that only 22% of the patients were eligible to receive CBCC after RNU and/or approximately 40% of patients did not receive a CBCC regimen at all [2]. Unfortunately, information about the chemotherapeutic regimen was not considered in our study, but we may speculate that CBCC regimen could not be administered to a considerable number of patients after RNU. We suggest that because patients will lose their kidney after RNU, prudent preoperative evaluation is necessary to predict which patients will benefit from neoadjuvant or adjuvant chemotherapy.
Our study has several limitations. First, the study was conducted with a retrospective design which warrants further prospective study. Second, data from multiple institutions could have several limitations, including variations among several surgeons and pathologists. Third, we did not examine the effect of lymph node status (pN0/pN+) which could have affected the oncologic outcomes. Fourth, we did not evaluate the HbA1c level in patients without a history of DM. In this perspective, our results might have been affected by selection bias because of the possibility of undiagnosed DM among patients classified as not having DM. In addition, we did not investigate the dose, type, duration of anti-diabetic medication, and the glycemic control status from RNU to recurrence and/or death which would affect the oncological outcomes, and there were no available data on the type of chemotherapeutic regimen. Further investigations with a prospective design, including the type and dose of anti-diabetic drug and type of chemotherapeutic regimen are needed to confirm our result. Despite these limitations, this is the first study to evaluate the impact of glucose control status using HbA1c on oncologic outcomes of patients with UTUC. Further study is necessary to elucidate the mechanism for the adverse effect of poor glycemic control status on UTUC patients.
In our study diabetic UTUC patients with poor preoperative glycemic control had significantly adverse oncologic outcomes compared with diabetic UTUC patients with good preoperative glycemic control and non-diabetics. CSS and OS of non-diabetic patients did not differ significantly from that of patients with good preoperative glycemic control. Rigorous diabetes control and monitoring using HbA1c is necessary to improve the prognosis of patients with DM and UTUC. Further well-designed prospective studies are needed to establish our findings.
ACKNOWLEDGMENTS
This study was supported by a grant from the Korean Urological Association Honam Section.
References
1. Dankner R, Balicer R, Boffetta P, Boker LK, Wallenstein S, Freedman L, et al. Diabetes, glucose control, glucose lowering medications, and cancer risk: a 10-year population-based historical cohort. BMC Cancer. 2012; 12:364.

2. Green DA, Rink M, Xylinas E, Matin SF, Stenzl A, Roupret M, et al. Urothelial carcinoma of the bladder and the upper tract: disparate twins. J Urol. 2013; 189:1214–21.

3. Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care. 2012; 35:1835–44.

4. Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008; 300:2754–64.
5. Hwang EC, Kim YJ, Hwang IS, Hwang JE, Jung SI, Kwon DD, et al. Impact of diabetes mellitus on recurrence and progression in patients with non-muscle invasive bladder carcinoma: a retrospective cohort study. Int J Urol. 2011; 18:769–76.

6. Oh JJ, Kang MY, Jo JK, Lee HM, Byun SS, Lee SE, et al. Association between diabetes mellitus and oncological outcomes in bladder cancer patients undergoing radical cystectomy. Int J Urol. 2015; 22:1112–7.

7. Rieken M, Xylinas E, Kluth L, Trinh QD, Lee RK, Fajkovic H, et al. Diabetes mellitus without metformin intake is associated with worse oncologic outcomes after radical nephroureterectomy for upper tract urothelial carcinoma. Eur J Surg Oncol. 2014; 40:113–20.

8. Joost HG. Diabetes and cancer: epidemiology and potential mechanisms. Diab Vasc Dis Res. 2014; 11:390–4.

9. Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004; 159:1160–7.

10. Emerging Risk Factors Collaboration, Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011; 364:829–41.

11. Tripathi A, Folsom AR, Anderson KE; Iowa Women's Health Study. Risk factors for urinary bladder carcinoma in postmenopausal women. The Iowa Women's Health Study. Cancer. 2002; 95:2316–23.
12. Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005; 293:194–202.

13. Hwang I, Jung SI, Nam DH, Hwang EC, Kang TW, Kwon DD, et al. Preoperative hydronephrosis and diabetes mellitus predict poor prognosis in upper urinary tract urothelial carcinoma. Can Urol Assoc J. 2013; 7:E215–20.

14. Siddiqui AA, Spechler SJ, Huerta S, Dredar S, Little BB, Cryer B. Elevated HbA1c is an independent predictor of aggressive clinical behavior in patients with colorectal cancer: a case-control study. Dig Dis Sci. 2008; 53:2486–94.

15. Lee H, Kuk H, Byun SS, Lee SE, Hong SK. Preoperative glycemic control status as a significant predictor of biochemical recurrence in prostate cancer patients after radical prostatectomy. PLoS One. 2015; 10:e0124761.

16. Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001; 131(11 Suppl):3109S–20S.

17. Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006; 160:1–40.

18. Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010; 33:1674–85.

19. Duan W, Shen X, Lei J, Xu Q, Yu Y, Li R, et al. Hyperglycemia, a neglected factor during cancer progression. Biomed Res Int. 2014; 2014:461917.

20. Liu S, Li Y, Lin T, Fan X, Liang Y, Heemann U. High dose human insulin and insulin glargine promote T24 bladder cancer cell proliferation via PI3K-independent activation of Akt. Diabetes Res Clin Pract. 2011; 91:177–82.

21. Metalli D, Lovat F, Tripodi F, Genua M, Xu SQ, Spinelli M, et al. The insulin-like growth factor receptor I promotes motility and invasion of bladder cancer cells through Akt- and mitogen-activated protein kinase-dependent activation of paxillin. Am J Pathol. 2010; 176:2997–3006.

22. Singh AK, Mo W, Dunea G, Arruda JA. Effect of glycated proteins on the matrix of glomerular epithelial cells. J Am Soc Nephrol. 1998; 9:802–10.

23. Krone CA, Ely JT. Controlling hyperglycemia as an adjunct to cancer therapy. Integr Cancer Ther. 2005; 4:25–31.

Fig. 1.
Kaplan-Meier plot for recurrence-free survival (A), cancer-specific survival (B), and overall survival (C) in upper tract urothelial carcinoma patients with no diabetes, good preoperative glycemic control, and poor preoperative glycemic control. (A) Recurrence-free survival (overall, p=0.002; no diabetes mellitus [DM] vs. hemoglobin A1c [HbA1c] < 7, p=0.05; no DM vs. HbA1c ≥ 7, p=0.011; HbA1c < 7 vs. HbA1c ≥ 7, p=0.001). (B) Cancer-specific survival (overall, p=0.001; no DM vs. HbA1c < 7, p=0.418; no DM vs. HbA1c < 7, p=0.418; HbA1c < 7 vs. HbA1c ≥ 7, p=0.001). (C) Overall survival (overall, p=0.001; no DM vs. HbA1c < 7, p=0.075; no DM vs. HbA1c ≥ 7, p=0.001; HbA1c < 7 vs. HbA1c ≥ 7, p=0.001).
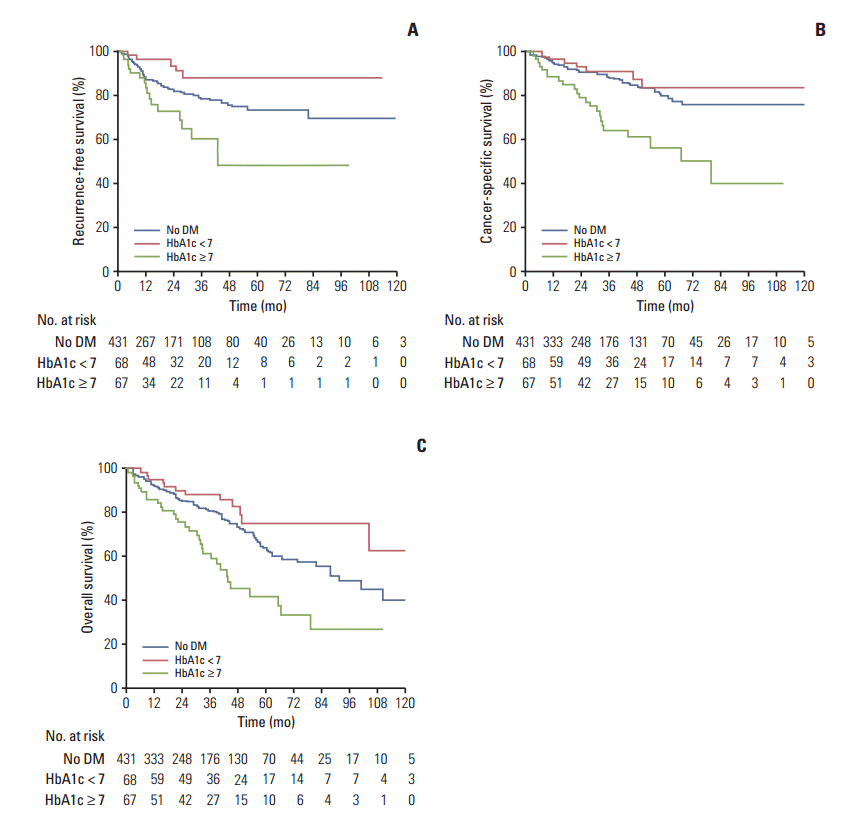
Table 1.
Baseline clinicopathological characteristics of the enrolled patients
| Variable | Total (n=566) | No DM (n=431) | DM, HbAlc < 7 (n=68) | DM, HbAlc ≥ 7 (n=67) | p-value |
|---|---|---|---|---|---|
| Age, median (IQR, yr) | 70 (62-75) | 70.5 (62-75) | 69.5 (63-69) | 68.8 (61-75) | 0.870a) |
| Sex | |||||
| Female | 165 (29.2) | 135 (31.3) | 17 (25) | 13 (19.4) | 0.098b) |
| Male | 401 (70.8) | 296 (68.7) | 51 (75) | 54 (80.6) | |
| Body mass index (kg/m2) | 23.8±3.1 | 23.6±3.0 | 24.9±3.6 | 23.8±2.8 | 0.049a) |
| ECOG PS | |||||
| 0 | 399 (70.5) | 314 (72.9) | 44 (64.7) | 41 (61.2) | 0.054b) |
| 1 | 146 (25.8) | 102 (23.7) | 21 (30.9) | 23 (34.3) | |
| 2 | 20 (3.5) | 15 (3.5) | 2 (2.9) | 3 (4.5) | |
| 3 | 1 (0.2) | 0 | 1 (1.5) | 0 | |
| Operation method | |||||
| Open | 142 (25.1) | 101 (23.4) | 17 (25.0) | 24 (35.8) | 0.094b) |
| Laparoscopic | 424 (74.9) | 330 (76.6) | 51 (75.0) | 43 (64.2) | |
| Tumor location | |||||
| Renal pelvis | 258 (45.6) | 195 (45.2) | 30 (44.1) | 33 (49.3) | 0.827b) |
| Upper ureter | 71 (12.5) | 51 (11.8) | 10 (14.7) | 10 (14.9) | |
| Mid ureter | 80 (14.1) | 60 (13.9) | 12 (17.6) | 8(11.9) | |
| Lower ureter | 157 (27.7) | 125 (29.0) | 16 (23.5) | 16 (23.9) | |
| Hydronephrosis | |||||
| None | 166 (29.3) | 119 (27.6) | 24 (35.3) | 23 (34.3) | 0.313b) |
| Mild | 151 (26.7) | 110 (25.5) | 22 (32.4) | 19 (28.4) | |
| Moderate | 146 (25.8) | 119 (27.6) | 14 (20.6) | 13 (19.4) | |
| Severe | 103 (18.2) | 83 (19.3) | 8(11.8) | 12 (17.9) | |
| Synchronous bladder tumor | |||||
| No | 445 (80.4) | 340 (78.9) | 58 (85.3) | 57 (85.1) | 0.274b) |
| Yes | 111 (19.6) | 91 (21.1) | 10 (14.7) | 10 (14.9) | |
| Tumor size (cm) | 3.6±2.5 | 3.6±2.5 | 3.2+2.2 | 3.6±2.3 | 0.297a) |
| Multifocality | |||||
| No | 517 (91.3) | 391 (90.7) | 63 (92.6) | 63 (94.0) | 0.615b) |
| Yes | 49 (8.7) | 40 (9.3) | 5 (7.4) | 4 (6.0) | |
| Pathologic stage | |||||
| Tis, Ta | 84 (14.8) | 66 (15.3) | 10 (14.7) | 8 (11.9) | 0.431b) |
| T1 | 128 (22.6) | 89 (20.6) | 21 (30.9) | 18 (26.9) | |
| T2 | 134 (23.7) | 104 (24.1) | 17 (25.0) | 13 (19.4) | |
| T3 | 200 (35.3) | 154 (35.7) | 19 (27.9) | 27 (40.3) | |
| T4 | 20 (3.5) | 18 (4.2) | 1 (1.5) | 1 (1.5) | |
| Pathologic grade | |||||
| Low | 178 (31.4) | 138 (32.0) | 23 (33.8) | 17 (25.4) | 0.499b) |
| High | 388 (68.6) | 293 (68.0) | 45 (66.2) | 50 (74.6) | |
| Lymphovascular invasion | |||||
| No | 447 (79.0) | 339 (78.7) | 58 (85.3) | 50 (74.6) | 0.297b) |
| Yes | 119 (21.0) | 92 (21.3) | 10 (14.7) | 17 (25.4) | |
| Concomitant CIS | |||||
| No | 512 (90.5) | 384 (89.1) | 67 (98.5) | 61 (91.0) | 0.048b) |
| Yes | 54 (9.5) | 47 (10.9) | 1 (1.5) | 6 (9.0) | |
| Adjuvant chemotherapy | |||||
| No | 361 (63.8) | 269 (62.4) | 49 (72.1) | 43 (64.2) | 0.306b) |
| Yes | 205 (36.2) | 162 (37.6) | 19 (27.9) | 24 (35.8) | |
| Recurrence | 92 (16.3) | 70 (16.2) | 5 (7.4) | 17 (25.4) | 0.018b) |
| Cancer death | 82 (14.5) | 52 (12.1) | 7 (10.3) | 23 (34.3) | 0.001b) |
| Death from any cause | 148 (26.1) | 103 (23.9) | 13 (19.1) | 32 (47.8) | 0.001b) |
Table 2.
Univariable Cox regression analyses predicting recurrence-free survival, cancer-specific survival, and overall survival
Table 3.
Multivariable Cox regression analyses predicting recurrence-free survival, cancer-specific survival, and overall survival




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download