Abstract
Purpose
Dexamethasone is a mainstay antiemetic regimen for the prevention of chemotherapy-induced nausea and vomiting. The aim of this pilot study was to assess the incidence of and factors associated with steroid-induced diabetes in cancer patients receiving chemotherapy with dexamethasone as an antiemetic.
Materials and Methods
Non-diabetic patients with newly diagnosed gastrointestinal cancer who received at least three cycles of highly or moderately emetogenic chemotherapy with dexamethasone as an antiemetic were enrolled. Fasting plasma glucose levels, 2-hour postprandial glucose levels, and hemoglobin A1C tests for the diagnosis of diabetes were performed before chemotherapy and at 3 and 6 months after the start of chemotherapy. The homeostasis model assessment of insulin resistance (HOMA-IR) was used as an index for measurement of insulin resistance, defined as a HOMA-IR ≥ 2.5.
Results
Between January 2012 and November 2013, 101 patients with no history of diabetes underwent laboratory tests for assessment of eligibility; 77 of these patients were included in the analysis. Forty-five patients (58.4%) were insulin resistant and 17 (22.1%) developed steroid-induced diabetes at 3 or 6 months after the first chemotherapy, which included dexamethasone as an antiemetic. Multivariate analysis showed significant association of the incidence of steroid-induced diabetes with the cumulative dose of dexamethasone (p=0.049).
Go to : 
Corticosteroids are often administered to cancer patients as a component of a chemotherapy regimen, to prevent chemotherapy-induced nausea and vomiting (CINV), to prevent allergic reactions caused by anti-cancer treatments, or as an adjuvant therapy with anti-edema effects [1-3]. Dexamethasone has a high therapeutic index when used for prevention of CINV, and a longer half-life and greater anti-inflammatory potency than other glucocorticoids [4]. According to several antiemetic guidelines, antineoplastic agents are grouped according to the risk of emesis they pose and should be matched to specific antiemetic regimens to reduce the degree of CINV; dexamethasone is generally used in combination with serotonin (5-hydroxytryptamine 3 [5-HT3]) or neurokinin-1 (NK-1) receptor antagonists for highly or moderately emetogenic chemotherapy, or as monotherapy for low-emetogenic chemotherapy in both the acute and delayed phases [5]. Antiemetic dexamethasone is often administered repeatedly over long periods of time in cancer patients receiving chemotherapy, which substantially increases the risk of adverse systemic effects [6]. The endocrine adverse effects of dexamethasone include adrenal suppression, hyperlipidemia, growth suppression, gynecomastia, and amenorrhea, with hyperglycemia as a frequently overlooked adverse effect of dexamethasone [2,3,7,8].
Glucocorticoids induce a state of relative insulin resistance. The effects of glucocorticoids on glucose metabolism include downregulation of glucose transporter 4 in muscle, which increases the amount of insulin needed for the uptake of glucose into cells, increased glucose production in the liver, inhibition of insulin binding to the insulin receptor on cells, and a decrease in insulin secretion from islet cells [9]. Therefore, glucocorticoid administration may exacerbate pre-diabetes or undiagnosed diabetes and can transform mild diabetes into a clinically severe illness, possibly leading to a hyperglycemic non-ketotic hyperosmolar coma. However, some of the symptoms of hyperglycemia, such as thirst, dry mouth, weakness, weight loss, and often polyuria and lethargy, are also common in patients with advanced malignancies for unrelated reasons such as the tumor itself, certain medicines, metabolic imbalance, and psychological problems. Therefore, the diagnosis of diabetes in cancer patients receiving chemotherapy is frequently delayed unless there is a high degree of clinical suspicion. However, the incidence of steroid-induced diabetes associated with the repeated use of the antiemetic dexamethasone for the prevention of CINV has not been reported to date.
Therefore, we designed this pilot study to assess the incidence of and factors associated with steroid-induced diabetes related to antiemetic dexamethasone therapy in cancer patients receiving chemotherapy for a prospective, multicenter clinical trial.
Go to : 
All consecutive eligible patients treated at the Department of Gastrointestinal Medical Oncology at Chungbuk National University Hospital were considered for this study. Chemotherapy-naïve patients with histologically confirmed cancer treated with highly or moderately emetogenic chemotherapy with antiemetic dexamethasone for at least 3 days per cycle were enrolled. Patients were required to have a life expectancy of ≥ 3 months and adequate hematologic, hepatic, and renal function. Patients with a history of diabetes or diabetic levels in laboratory tests before chemotherapy, a history of pancreatic cancer, had received corticosteroids except antiemetic dexamethasone within 6 months of study commencement, or had a serious concurrent infection or nonmalignant illness were excluded from the analysis.
All patients provided written informed consent to participate in the study. This study was reviewed and approved by the Institutional Review Board of Chungbuk National University Hospital.
According to the National Comprehensive Cancer Network guidelines, all patients treated with highly emetogenic chemotherapy received 125 mg of an oral aprepitant plus a 5-HT3 receptor antagonist and 10-12 mg of dexamethasone on day 1, 80 mg of an oral aprepitant and 7-8 mg of daily oral dexamethasone on days 2 and 3, and 7-8 mg of dexamethasone on day 4. Patients treated with moderately emetogenic chemotherapy received a 5-HT3 receptor antagonist and 10-12 mg of dexamethasone on day 1, followed by 7-8 mg of daily dexamethasone on days 2-3 [5]. These antiemetics were administered every 2-4 weeks according to the chemotherapy schedule.
Laboratory tests for diagnosis of diabetes and insulin resistance were performed before chemotherapy and at 3 and 6 months after the start of chemotherapy. All patients fasted for more than 8 hours before blood collection for measurement of fasting plasma glucose (FPG), hemoglobin A1C (HbA1C), insulin, and serum C-peptide levels. Blood samples were drawn again to determine 2-hour postprandial glucose (PP2) levels exactly 2 hours after eating a meal. To minimize the direct effect of chemotherapeutic agents and transient hyperglycemia induced by dexamethasone, all blood tests were performed before the chemotherapy session. A diagnosis of diabetes was based on the following: FPG ≥ 7 mmol/L (126 mg/dL), PP2 ≥ 11.1 mmol/L (200 mg/dL), or HbA1C ≥ 6.5% [10]. Patients showing diabetic levels in laboratory tests performed before the start of chemotherapy were excluded. The homeostasis model assessment for insulin resistance (HOMA-IR) was calculated using the following formula: FPG (mmol/L)×fasting insulin (μIU/L)/22.5 [11]. Insulin resistance was defined as a HOMA-IR ≥ 2.5 [12,13].
Mean values were compared using the t test (two categories) and one-way analysis of variance (more than two categories). Proportions were compared using two-way tables and χ2 tests. Univariate and multivariate regression analyses were performed to examine the association between the incidence of diabetes and clinical variables. Potential explanatory variables were age (< 60 years or ≥ 60 years), sex (male or female), Eastern Cooperative Oncology Group (ECOG) performance status (0, 1, or 2), body mass index (< 20 kg/m2, 20-25 kg/m2, or ≥ 25 kg/m2), primary tumor site (esophagus/hepatobiliary, stomach, or colorectal), stage (III or IV), intent of first chemotherapy (adjuvant or palliative), emetic risk of first chemotherapy (high or moderate), use of megestrol acetate (yes or no), HOMA-IR (< 2.50 or ≥ 2.50), and cumulative dose of dexamethasone (< 156 mg or ≥ 156 mg). For logistic regression analysis, high dexamethasone usage was defined as a cumulative dose of dexamethasone ≥ 156 mg, which was equivalent to six cycles of moderately emetogenic chemotherapy that included dexamethasone as an antiemetic. p < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL).
Go to : 
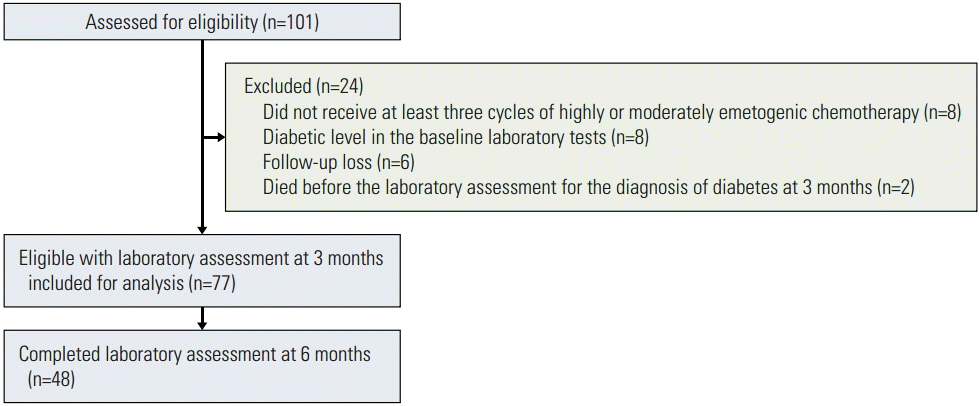
Between January 2012 and November 2013, 101 patients with no history of diabetes underwent laboratory assessment to determine their eligibility for inclusion in the study; of these, 24 were excluded for the following reasons: less than three cycles of highly or moderately emetogenic chemotherapy (eight patients), a diabetic level in baseline FPG, PP2, and HbA1C tests (eight patients), lost to follow-up (six patients), or died before laboratory assessment at 3 months after the first chemotherapy (two patients). Consequently, 77 patients who met the above criteria were included in the analysis (Fig. 1).
The baseline characteristics of these 77 patients are listed in Table 1. The median age was 59 years (range, 36 to 81 years). Fifty-five patients (71.4%) were male, and 69 (89.6%) had good performance status (ECOG 0-1). Seventy-seven patients (93.5%) had gastric or colorectal cancer. Fifty patients (64.9%) received palliative chemotherapy and 44 (57.1%) received highly emetogenic chemotherapy. Thirteen patients (16.9%) received megestrol acetate to relieve symptoms of anorexia during the study period.
Patient characteristics
The incidence of insulin resistance and diabetes is shown in Table 2. Insulin resistance was detected in 22 non-diabetic patients (28.6%) before the first chemotherapy and in 45 of the 77 patients (58.4%) at 3 or 6 months after the first chemotherapy. Steroid-induced diabetes was detected at 3 or 6 months after the first chemotherapy, which included dexamethasone as an antiemetic in 17 patients (22.1%). In the laboratory tests for the diagnosis of diabetes, diabetic levels were detected in 11 of the 77 patients (14.3%) tested at 3 months after the start of the first chemotherapy and in eight of the 48 patients (16.7%) tested at 6 months.
Incidence of insulin resistance and diabetes, as determined by the laboratory tests
A comparison of the laboratory results for diabetic and non-diabetic patients at 3 or 6 months after the start of chemotherapy is shown in Table 3. No significant differences in terms of baseline FPG, PP2, HbA1C, and C-peptide levels were observed between the groups. However, a significant difference in the baseline HOMA-IR was observed between non-diabetic and diabetic patients (2.16±1.42 vs. 3.44±5.52, respectively; p=0.023). Significant differences with respect to PP2 (6.41±1.63 mmol/L vs. 9.19±2.68 mmol/L, respectively; p=0.003) and HOMA-IR (3.07 ± 2.06 mmol/L vs. 5.03 ± 5.13 mmol/L, respectively; p=0.001) were also observed between non-diabetic and diabetic patients at 3 months. At 6 months after the start of the first chemotherapy, there were significant differences between all laboratory test results for the two groups: FPG (5.28±0.60 mmol/L vs. 5.93±1.11 mmol/L, p=0.004), PP2 (6.22±1.46 mmol/L vs. 9.09±3.43 mmol/L, p < 0.001), HbA1C (5.39±0.46% vs. 6.30±1.05%, p=0.016), C-peptide (2.32±0.03 ng/mL vs. 4.91±3.88 ng/mL, p=0.003), and HOMA-IR (2.37±1.81 mmol/L vs. 6.04±6.37 mmol/L, p=0.001).
Results of the laboratory assessment according to the diagnosis of diabetes
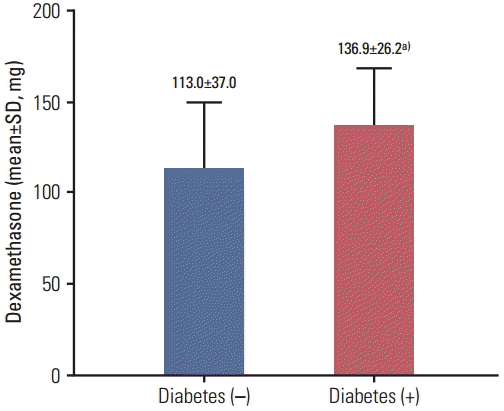
The incidence of diabetes according to clinical variables is shown in Table 4. In the univariate analysis, the incidence of diabetes was significantly affected by age, sex, and the cumulative dose of dexamethasone. The incidence of diabetes was 12.8% in patients < 60 years of age and 31.6% in those ≥ 60 years of age (p=0.032), and 29.1% in men and 4.5% in women (p=0.013). The cumulative dose of dexamethasone was a significant factor affecting the incidence of diabetes; patients who developed diabetes had received a larger cumulative dose of dexamethasone than those who had not (mean±standard deviation, 136.9±26.2 vs. 113.0±37.0 mg; p=0.015) (Fig. 2), and the incidence of steroid-induced diabetes in patients receiving cumulative doses of dexamethasone < 156 mg and ≥ 156 mg was 9.1% and 31.8%, respectively (p=0.025).
Univariate and multivariate analyses of clinical characteristics associated with diabetes
In multivariate analysis the cumulative dose of dexamethasone was the only significant factor for the incidence of steroid-induced diabetes associated with antiemetic dexamethasone therapy (p=0.049) (Table 4).
Go to : 
Many chemotherapeutic regimens include corticosteroids which may induce diabetes or exacerbate pre-existing diabetes [1-3,14,15]. Yoo et al. [14], who examined 632 patients who received docetaxel chemotherapy and underwent screening for hyperglycemia, reported that the incidence of hyperglycemia was 13.8% and the risk factors for hyperglycemia were being overweight or obese, and a history of diabetes mellitus. Brunello et al. [15] reviewed 349 non-Hodgkin lymphoma and prostate cancer patients treated with a chemotherapy regimen inclusive of a steroid. Abnormal glucose levels at baseline were detected in 44% and 68% of patients with non-Hodgkin lymphoma and prostate cancer, and dysglycemia was detected in 70% of patients with non-Hodgkin lymphoma and 92% of patients with prostate cancer over the course of treatment. However, despite the fact that dexamethasone is the most frequently used antiemetic for the prevention of CINV in cancer patients receiving chemotherapy, hyperglycemia after antiemetic dexamethasone therapy itself has not been reported to date. The aim of this pilot study was to assess the incidence of steroid-induced diabetes after antiemetic dexamethasone therapy in cancer patients for a prospective, multicenter clinical trial. We suggest that the incidence of steroid-induced diabetes in non-diabetic cancer patients receiving chemotherapy with antiemetic dexamethasone therapy was approximately 20%; this result was significantly affected by the cumulative dose of dexamethasone.
Corticosteroids induce a state of relative insulin resistance and primarily cause postprandial hyperglycemia [2,9]. Insulin resistance is a condition characterized by reduced tissue responses to the action of insulin, which results in hyperglycemia and hyperinsulinemia. HOMA-IR, which is based on FPG and insulin levels, has been widely validated and used as a measure of insulin resistance in large epidemiologic studies and in clinical practice [11]. Here, insulin resistance (as determined by HOMA-IR) was detected in 45 of 77 patients (58.4%) at 3 or 6 months after the first chemotherapy with antiemetic dexamethasone. In addition, a significant difference in the HOMA-IR was observed between the non-diabetic and diabetic patients after 3 or 6 months of repetitive use of antiemetic dexamethasone. There was also a significant difference in the baseline HOMA-IR before the first chemotherapy. Therefore, although HOMA-IR did not show significant association with steroid-induced diabetes in multivariate analysis, the probability of developing diabetes after antiemetic dexamethasone therapy is higher in patients who are already insulin resistant prior to the first chemotherapy with antiemetic dexamethasone. C-peptide is co-secreted on an equimolar basis from beta cells, it is not extracted or metabolized by the liver, and therefore is a more accurate indicator of pancreatic insulin secretion than insulin itself [16]. No significant difference in C-peptide levels was observed between non-diabetic and diabetic patients at baseline or at 3 months; however, C-peptide levels at 6 months after the start of chemotherapy were significantly higher in diabetic patients than in non-diabetic patients. Therefore, it could be suggested that the longer a patient receives antiemetic dexamethasone, the more insulin is secreted by the pancreas.
A typical patient with hyperglycemia after corticosteroid therapy will have elevated glucose values 1-2 hours after a meal, which drop to normal overnight. Postprandial hyperglycemia after corticosteroid therapy is particularly evident after morning doses of corticosteroid, and could in part be related to the effects of steroid wearing off overnight; however, an overnight improvement in glucose levels is also seen with longer-acting dexamethasone [2]. The current study only found a difference in PP2 levels between non-diabetic and diabetic patients at 3 months, but significant differences in FPG, PP2, and HbA1C levels were observed at 6 months after the start of chemotherapy with antiemetic dexamethasone. In addition, in the current study blood tests were performed before the administration of chemotherapy including antiemetic dexamethasone in order to minimize the transient hyperglycemia induced by dexamethasone. Therefore, in our study the incidence of steroid-induced diabetes after antiemetic dexamethasone therapy was not transient hyperglycemia but true diabetes, and the incidence of hyperglycemia itself would have been higher if transient hyperglycemia within a few days after dexamethasone administration had been included.
The clinician may not detect steroid-induced hyperglycemia in cancer patients, because hyperglycemia itself is asymptomatic unless pronounced, leading to thirst, dry mouth, and often polyuria and lethargy. Even if hyperglycemia is symptomatic, some of the symptoms are also common in patients with advanced malignancies; therefore, the diagnosis of diabetes is frequently delayed unless there is a high degree of clinical suspicion. However, hyperglycemia may lead to acute complications or adverse events, such as dehydration, ketoacidosis, and acute hyperglycemic syndrome [17], and it has a negative impact on survival in cancer patients. Recent large cohort studies suggest an association of pre-existing diabetes at the time of cancer diagnosis with increased all-cause mortality and cancer recurrence, particularly in patients with colon and breast cancers [18-21]. Hyperglycemia had a significant effect on survival in patients with lung cancer [22,23] and acute leukemia [17]. There are several explanations for the association between diabetes and increased all-cause mortality in cancer patients. First, cancer patients with diabetes may show increased tumor cell proliferation and metastases associated with the hyperinsulinemic environment. High insulin levels or an increase in free insulin-like growth factor levels may promote cancer cell proliferation and tumor growth [24]. Second, the association between hyperglycemia and poor survival may be mediated by adverse effects of hyperglycemia unrelated to tumor growth, such as increased rates of infection, which may delay and decrease the efficacy of treatment and thus directly affect survival [25]. Therefore, cancer patients with no history of diabetes who are treated with corticosteroids should be monitored intermittently for hyperglycemia.
In the current study, high doses of antiemetic dexamethasone were significantly associated with the development of steroid-induced diabetes. Patients who developed diabetes during the 6 months of treatment received a larger cumulative dose of dexamethasone than those who had not. Diabetes occurred in approximately 32% of the patients who received more than 156 mg dexamethasone, which was delivered in six cycles of moderately emetogenic chemotherapy. Systemic chemotherapy in cancer has improved remarkably over recent years, resulting in increased survival rates and a larger number of cancer patients receiving longer courses of chemotherapy. To prevent or reduce CINV, dexamethasone is administered repeatedly over long periods of time to cancer patients receiving chemotherapy, which substantially increases the risk of steroid-induced diabetes. Further prospective studies are needed to determine the optimal dose and duration of antiemetic dexamethasone therapy to achieve a reduction in the incidence of dexamethasone-related adverse effects such as steroid diabetes while controlling CINV in patients treated with long-term chemotherapy, particularly those receiving chemotherapy for palliative intent.
The current study has several limitations. First, it was intended to assess the incidence of and risk factors for steroid-induced diabetes, and was not powered to determine the effect of steroid-induced diabetes or insulin resistance. Second, our population was limited to patients with gastrointestinal cancer; therefore, our findings may not apply to patients receiving dexamethasone as an antiemetic for other types of cancer. Third, we do not know the exact rate of compliance with dexamethasone therapy subsequent to the third cycle of chemotherapy because compliance with oral dexamethasone therapy on days 2, 3, or 4 may have been low after the first cycle if the patient did not develop CINV. Last, the number of patients recruited to the study was relatively small because it was designed as a pilot study prior to a prospective, multicenter clinical trial. The relatively small sample size resulted in insufficient statistical power for detailed subgroup analyses.
Go to : 
In conclusion, the current study suggests that approximately 20% of non-diabetic cancer patients receiving chemotherapy combined with the antiemetic dexamethasone for the prevention of CINV develop steroid-induced diabetes; this was particularly significant for patients receiving repetitive chemotherapy with high cumulative doses of dexamethasone. To optimize patient care and outcomes, patients receiving chemotherapy with antiemetic dexamethasone should receive adequate support and monitoring to prevent the development of dexamethasone-induced diabetes and its associated complications. We suggest conduct of a larger, prospective, multicenter clinical trial to confirm these findings and to assess the effect of steroid-induced diabetes on the efficacy of chemotherapy, infection-related adverse events, and patient survival.
Go to : 
ACKNOWLEDGMENTS
This research was supported by a Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (2007-0054930).
Go to : 
References
1. Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009; 16:1103–23.

2. Oyer DS, Shah A, Bettenhausen S. How to manage steroid diabetes in the patient with cancer. J Support Oncol. 2006; 4:479–83.
3. Poulson J. The management of diabetes in patients with advanced cancer. J Pain Symptom Manage. 1997; 13:339–46.

5. NCCN Clinical Practice Guidelines in Oncology: antiemesis. Version 2, 2014 [Internet]. Fort Washington, PA: National Comprehensive Cancer Network;2014 [cited 2015 Mar 1]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf.
6. Vardy J, Chiew KS, Galica J, Pond GR, Tannock IF. Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer. 2006; 94:1011–5.

7. Fardet L, Kassar A, Cabane J, Flahault A. Corticosteroid-induced adverse events in adults: frequency, screening and prevention. Drug Saf. 2007; 30:861–81.
8. Han HS, Park JC, Park SY, Lee KT, Bae SB, Kim HJ, et al. A prospective multicenter study evaluating secondary adrenal suppression after antiemetic dexamethasone therapy in ccancer patients receiving chemotherapy: a Korean South West Oncology Group Study. Oncologist. 2015; 20:1432–9.
9. Rafacho A, Ortsater H, Nadal A, Quesada I. Glucocorticoid treatment and endocrine pancreas function: implications for glucose homeostasis, insulin resistance and diabetes. J Endocrinol. 2014; 223:R49–62.

10. Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J. Harrison’s principles of internal medicine. 18th ed. New York: McGraw-Hill Companies, Inc.;2011.
11. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–9.
12. Kim CH, Kim HK, Kim EH, Bae SJ, Park JY. Relative contributions of insulin resistance and beta-cell dysfunction to the development of Type 2 diabetes in Koreans. Diabet Med. 2013; 30:1075–9.
13. Son JW, Park CY, Kim S, Lee HK, Lee YS; Insulin Resistance as Primary Pathogenesis in Newly Diagnosed, Drug Naïve Type 2 Diabetes Patients in Korea (SURPRISE) Study Group. Changing clinical characteristics according to insulin resistance and insulin secretion in newly diagnosed type 2 diabetic patients in Korea. Diabetes Metab J. 2015; 39:387–94.

14. Yoo KE, Kang RY, Lee JY, Lee YJ, Suh SY, Kim KS, et al. Awareness of the adverse effects associated with prophylactic corticosteroid use during docetaxel therapy. Support Care Cancer. 2015; 23:1969–77.

15. Brunello A, Kapoor R, Extermann M. Hyperglycemia during chemotherapy for hematologic and solid tumors is correlated with increased toxicity. Am J Clin Oncol. 2011; 34:292–6.

16. Polonsky KS, Rubenstein AH. C-peptide as a measure of the secretion and hepatic extraction of insulin. Pitfalls and limitations. Diabetes. 1984; 33:486–94.

17. Weiser MA, Cabanillas ME, Konopleva M, Thomas DA, Pierce SA, Escalante CP, et al. Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate-cytarabine regimen. Cancer. 2004; 100:1179–85.

18. Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB 3rd, et al. Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol. 2003; 21:433–40.

19. van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, Dercksen MW, Coebergh JW, Haak HR. Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. Int J Cancer. 2007; 120:1986–92.

20. Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008; 300:2754–64.
21. Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001; 285:885–92.

22. Luo J, Chen YJ, Chang LJ. Fasting blood glucose level and prognosis in non-small cell lung cancer (NSCLC) patients. Lung Cancer. 2012; 76:242–7.

23. Nakazawa K, Kurishima K, Tamura T, Ishikawa H, Satoh H, Hizawa N. Survival difference in NSCLC and SCLC patients with diabetes mellitus according to the first-line therapy. Med Oncol. 2013; 30:367.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download