This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
Previous studies examining the relationship between time to treatment and survival outcome in breast cancer have shown inconsistent results. The aim of this study was to analyze the overall impact of delay of treatment initiation on patient survival and to determine whether certain subgroups require more prompt initiation of treatment.
Materials and Methods
This study is a retrospective analysis of stage I-III patients who were treated in a single tertiary institution between 2005 and 2008. Kaplan-Meier survival analysis and Cox proportional hazards regression model were used to evaluate the impact of interval between diagnosis and treatment initiation in breast cancer and various subgroups.
Results
A total of 1,702 patients were included. Factors associated with longer delay of treatment initiation were diagnosis at another hospital, medical comorbidities, and procedures performed before admission for surgery. An interval between diagnosis and treatment initiation as a continuous variable or with a cutoff value of 15, 30, 45, and 60 days had no impact on disease-free survival (DFS). Subgroup analyses for hormone-responsiveness, triple-negative breast cancer, young age, clinical stage, and type of initial treatment showed no significant association between longer delay of treatment initiation and DFS.
Conclusion
Our results show that an interval between diagnosis and treatment initiation of 60 days or shorter does not appear to adversely affect DFS in breast cancer.
Go to :

Keywords: Breast neoplasms, Time-to-treatment, Survival rate
Introduction
While starting treatment for breast cancer without delay is theoretically ideal, there are no established guidelines regarding what in practice constitutes an acceptable interval between the diagnosis of breast cancer and treatment initiation. Many factors may contribute to the delay of treatment initiation and although a number of studies have been conducted to assess what influence this might have on patient survival, their results have been conflicting [
1-
6].
Regardless of its cause, delay of treatment initiation causes great anxiety to patients and their families. According to a study examining the quality of life across the continuum of breast cancer care, the most anxiety-provoking time for patients is the waiting period for treatment initiation after diagnosis [
7]. Most patients fear that their cancer will progress during this time and prolonged delay of treatment initiation can also cause concern to the treating physician. Knowing the potential influence of delay of treatment initiation on patient survival, and distinguishing those patients who require more timely treatment can be clinically valuable.
Through this study, we sought to investigate demographic and clinical pathological factors associated to delay of treatment initiation and to assess the impact of delay of treatment initiation on patient survival and identify which subgroup(s) of patients require more prompt treatment initiation.
Go to :

Materials and Methods
A retrospective review of patients who underwent surgery for breast cancer at Seoul National University Hospital (SNUH) between July 2005 and June 2008 was performed. Basic clinicopathological data were extracted from SNUH Breast Care Center database, which is a prospectively maintained web-based database, and “event” data were reviewed by the first author using the electronic medical records. Survival data was obtained from the Korean National Statistical Office database. Patients with invasive breast cancer who started their initial treatment at SNUH and for whom either the date of pathological diagnosis or date of referral was known were included. Patients who underwent surgery for in-situ carcinoma, those who underwent palliative operations (including patients diagnosed with distant metastases within 4 months of diagnosis), patients who did not have adjuvant therapy data or those who refused recommended adjuvant treatment were excluded. Patients who received neoadjuvant chemotherapy were excluded as these patients have different clinicopathologic characteristics compared to patients undergoing surgery as initial treatment. Also patients with a treatment delay of 6 months or greater were excluded, presuming that such unusually long intervals would be due to a patient’s, or their family’s refusal of standard treatment, which was not the main concern of this study.
Interval between diagnosis and treatment initiation was defined as time between date of pathological diagnosis and start of treatment. Pathological diagnosis was made by core needle biopsy or fine needle aspiration. Where pathological diagnosis had been made in another institution and therefore date was unknown, date of referral from the other hospital was used instead as there normally is only 2-3 days difference in these two dates. Where both dates were unknown, the patient was excluded from the study.
Patient-level socio-demographic variables included age at diagnosis, marital status, district of residence, comorbidities, hospital of diagnosis, presence of breast cancer-related symptoms at diagnosis, and family history of cancer. District of residence was categorized according to either Seoul-Incheon (Capital) area and its’ satellite cities, or outside the Capital area.
Tumor-specific characteristics included: tumor size, axillary lymph node metastasis status, cancer stage, histologic grade, tumor hormone receptor status, and human epidermal growth factor receptor 2 (HER2) status. Clinical stage was determined by physical examination and referring hospital imaging results which were assessed at the patient’s first visit to SNUH outpatient clinic. Pathological breast cancer staging was defined according to the 7th edition of the American Joint Committee on Cancer.
Clinical characteristics included factors that can delay treatment initiation such as the need for an additional biopsy, preoperative imaging studies performed prior to admission, clinical consultation with other departments due to comorbidities, hospitalization prior to surgery, and immediate breast reconstruction. Imaging studies were categorized according to routine staging work-up (chest computed tomography, bone scan, breast magnetic resonance imaging, or positron emission tomography computed tomography) versus non-routine imaging.
The primary endpoint was disease-free survival (DFS). DFS was calculated from the time of treatment initiation to either the date of breast cancer recurrence or the final outpatient clinic visit. Breast cancer recurrence included locoregional recurrence and distant metastases. Secondary endpoint was overall survival (OS), defined as date of treatment initiation to date of expire or date of last out-patient clinic visit. Analyses were performed to assess the relationship between baseline characteristics with the length of interval between diagnosis and treatment initiation, using chi-square test and t test. In addition the impact of interval length on DFS and OS was evaluated using Kaplan-Meier survival analysis and log-rank test. Multivariate survival analysis adjusting clinicopathologic factors that are known to affect patients’ survival, including age, tumor size, lymph node metastasis, histologic grade, and hormone receptor status, was performed using a Cox proportional hazards regression model. For subgroup analysis, interval between diagnosis and treatment initiation was dichotomized into two groups (0 to 29 and ≥ 30 days).
This study was approved by the Institutional Review Board of SNUH and the committee waived the requirement for informed consent.
Go to :

Results
A total of 2,256 patients underwent curative surgery for invasive breast cancer at SNUH from July 2005 to June 2008; 554 patients were excluded from the study, including 234 patients who received neoadjuvant chemotherapy and 264 patients whose date of pathological diagnosis or referral date from another hospital was unknown. The mean age of the 1,702 patients who were included in the study was 48.0 years. Their median interval between diagnosis and treatment initiation was 23 days (range, 0 to 134 days); 66.6% of women received initial treatment within 30 days of diagnosis and 1.8% received initial treatment more than 60 days after their diagnosis. The distribution of interval between diagnosis and treatment initiation is shown in
Fig. 1.
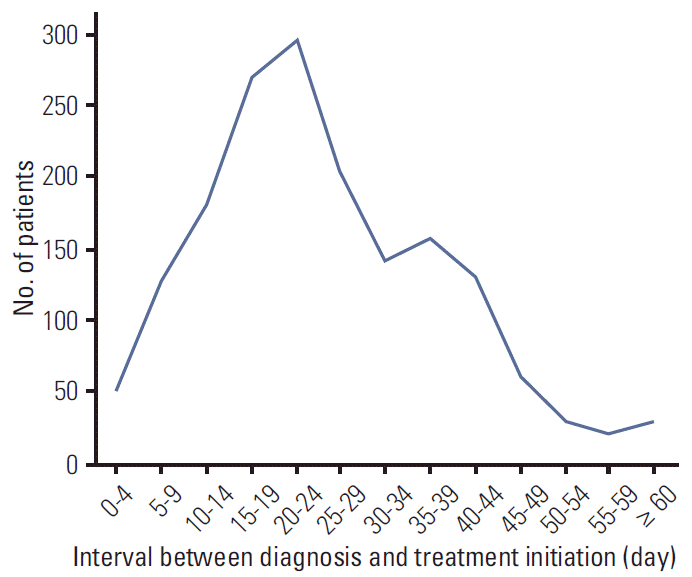 | Fig. 1.Distribution of interval between diagnosis and treatment initiation. 
|
Various factors were associated with longer interval between diagnosis and treatment initiation. Demographic characteristics significantly associated with longer interval of ≥ 30 days were diagnosis at another hospital (p < 0.001) and medical comorbidities (p=0.015). In addition, interval between diagnosis and treatment initiation was significantly longer for women who underwent imaging studies prior to admission for surgery (p < 0.001), those who required an additional biopsy (p < 0.001), those who required clinical consultation with other departments (p < 0.001), and those who required hospitalization prior to treatment initiation (p < 0.001). Age or immediate reconstructive surgery were not associated with longer interval between diagnosis and treatment initiation (p > 0.05).
Clinical stage and pathological stage did not differ according to interval to treatment initiation. Patients with hormone receptor-positive tumors had longer intervals between diagnosis and treatment initiation (p=0.043). Treatment related factors associated with longer intervals were adjuvant endocrine therapy (p=0.023) and adjuvant chemotherapy (p=0.004) (
Table 1).
Table 1.
Socio-demographic, clinical, and tumor-specific characteristics associated with interval between diagnosis and treatment initiation of ≥ 30 days
|
Factor |
Interval of 0 to 29 days |
Interval of > 30 days |
p-valuea)
|
|
Total (n=1,702)
|
1,133 (66.6) |
569 (33.4) |
|
|
Age (yr)
|
|
|
|
|
≤ 39 |
180 (15.9) |
74 (13.0) |
0.336 |
|
40-49 |
478 (42.2) |
241 (42.4) |
|
|
50-59 |
320 (28.2) |
160 (28.1) |
|
|
60-69 |
124 (10.9) |
78 (13.7) |
|
|
≥ 70 |
31 (2.7) |
16 (2.8) |
|
|
Address
|
|
|
|
|
Seoul-Incheon area |
795 (70.2) |
398 (69.9) |
0.925 |
|
Outside of capital area |
338 (29.8) |
171 (30.1) |
|
|
Place of diagnosis
|
|
|
|
|
SNUH |
494 (43.6) |
195 (34.3) |
< 0.001 |
|
Other hospital |
639 (56.4) |
374 (65.7) |
|
|
Education
|
|
|
|
|
≤ High school |
636 (56.1) |
350 (61.5) |
0.091 |
|
> High school |
443 (39.1) |
92 (33.7) |
|
|
Unknown |
54 (4.8) |
27 (4.7) |
|
|
Marital status
|
|
|
|
|
Married |
1,033 (91.2) |
516 (90.7) |
0.621 |
|
Single |
60 (5.3) |
38 (6.7) |
|
|
Divorced |
9 (0.8) |
3 (0.5) |
|
|
Widowed |
7(0.6) |
4 (0.7) |
|
|
Unknown |
24 (2.1) |
8 (1.4) |
|
|
Comorbidity
|
|
|
|
|
Other than cancer |
306 (27.0) |
186 (32.7) |
0.015 |
|
No comorbidities |
827 (73.0) |
383 (67.3) |
|
|
Symptom
|
|
|
|
|
Yes |
761 (67.2) |
362 (63.6) |
0.155 |
|
No |
369 (32.6) |
207 (36.4) |
|
|
Unknown |
3 (0.3) |
0| |
|
|
Cancer family history
|
|
|
|
|
Breast cancer related |
101 (8.9) |
43 (7.5) |
0.609 |
|
Not related to breast cancer |
173 (15.3) |
85 (14.9) |
|
|
No cancer family history |
859 (75.8) |
441 (77.5) |
|
|
Additional biopsy
|
|
|
|
|
Any |
36 (3.2) |
62 (10.9) |
< 0.001 |
|
None |
1,097 (96.8) |
507 (89.1) |
|
|
Imaging before admission
|
|
|
|
|
Any |
424 (37.4) |
340 (59.8) |
< 0.001 |
|
None |
709 (62.6) |
229 (40.2) |
|
|
Not routine imaging before admission
|
|
|
|
|
Any |
5 (0.4) |
27 (4.7) |
< 0.001 |
|
None |
1,128 (99.6) |
542 (95.3) |
|
|
Hospitalization
|
|
|
|
|
Any |
2 (0.2) |
11 (1.9) |
< 0.001 |
|
None |
1,131 (99.8) |
558 (98.1) |
|
|
Consultation
|
|
|
|
|
Any |
48 (4.2) |
56 (9.8) |
< 0.001 |
|
None |
1,085 (95.8) |
513 (90.2) |
|
|
Immediate reconstruction
|
|
|
|
|
Yes |
11 (1.0) |
6 (1.1) |
0.870 |
|
No |
1,122 (99.0) |
563 (98.9) |
|
|
Clinical stage at diagnosis
|
|
|
|
|
Diagnosis by FNA |
29 (2.6) |
14 (2.5) |
0.109 |
|
In situ cancer |
91 (8.0) |
59 (10.4) |
|
|
T1N0 |
681 (60.1) |
357 (62.7) |
|
|
≥ T2 or LN(+) |
332 (29.3) |
139 (24.4) |
|
|
Pathological stage
|
|
|
|
|
T size ≤ 2 cm |
625 (55.2) |
327 (57.5) |
0.366 |
|
T size > 2 cm |
508 (44.8) |
242 (42.5) |
|
|
No ALN metastasis |
727 (64.2) |
387 (68.0) |
0.115 |
|
ALN metastasis |
406 (35.8) |
182 (32.0) |
|
|
Histologic grade
|
|
|
|
|
Grade 1, 2 |
527 (50.6) |
290 (55.3) |
0.075 |
|
Grade 3 |
515 (59.4) |
234 (44.7) |
|
|
Hormone receptor status
|
|
|
|
|
Positive |
764 (67.4) |
411 (72.2) |
0.043 |
|
Negative |
369 (32.6) |
158 (27.8) |
|
|
Ki-67
|
|
|
|
|
Low (< 10%) |
892 (78.9) |
452 (79.7) |
0.709 |
|
High (≥ 10%) |
238 (21.1) |
115 (20.3) |
|
|
Radiotherapy
|
|
|
|
|
Yes |
761 (67.2) |
385 (67.7) |
0.837 |
|
No |
372 (32.8) |
184 (32.3) |
|
|
Chemotherapy
|
|
|
|
|
Yes |
869 (76.7) |
400 (70.3) |
0.004 |
|
No |
264 (23.3) |
169 (29.7) |
|
|
Endocrine therapy
|
|
|
|
|
Yes |
757 (66.8) |
411 (72.2) |
0.023 |
|
No |
376 (33.2) |
158 (27.8) |
|

The median duration of follow up was 5.9 years. 5-Year OS and DFS rate were 95.9% and 91.3%, respectively. An interval of 15, 30 days had no impact on DFS by univariate and multivariate analysis (p=0.079 and p=0.101, respectively) (
Table 2,
Fig. 2A and
B). In addition, a longer interval of 45 or 60 days had no impact on DFS (p=0.431 and p=0.839, respectively) (
Fig. 2C and
D), and an interval as a continuous variable also had no significant influence (p=0.093). Regarding OS, no significant association between an interval of ≥ 30 days was demonstrated (p=0.952) (
Fig. 3).
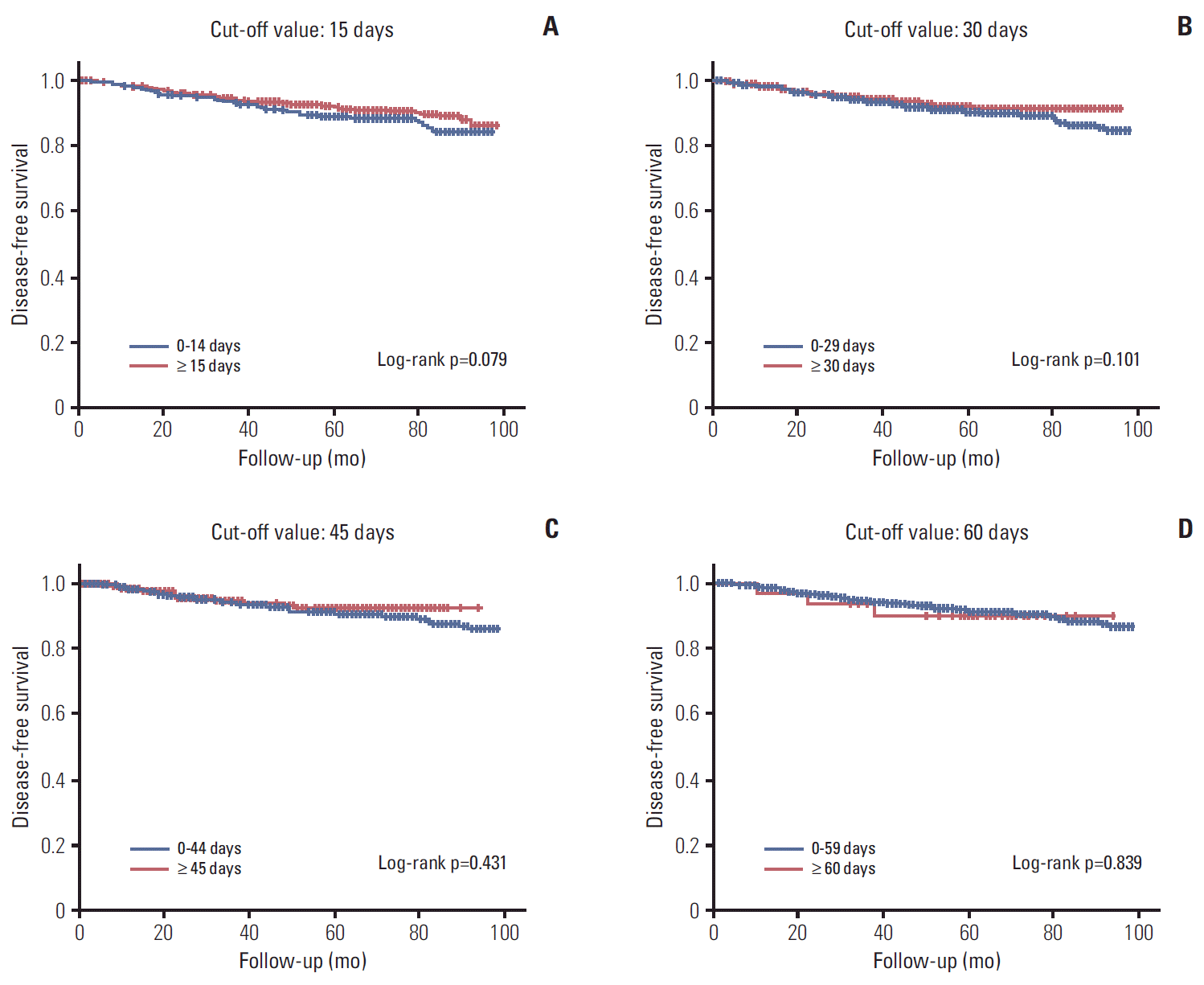 | Fig. 2.Kaplan-Meier survival curves of disease-free survival (DFS) by interval between diagnosis and treatment. (A) DFS by interval of ≥ 15 days versus 0-14 days. (B) DFS by interval of ≥ 30 days versus 0-29 days. (C) DFS by interval of ≥ 45 days versus 0-44 days. (D) DFS by interval of ≥ 60 days versus 0-59 days. 
|
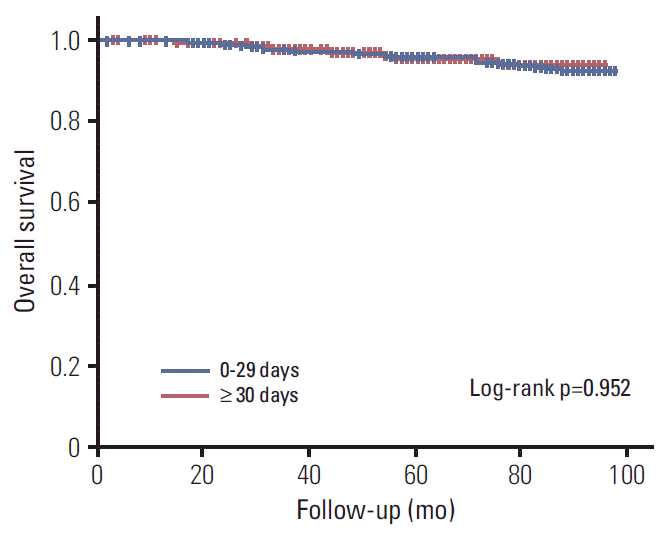 | Fig. 3.Kaplan-Meier survival curves of overall survival by interval of ≥ 30 days versus 0-29 days. 
|
Table 2.
Multivariate analysis of factors affecting disease-free survival for interval between diagnosis and treatment initiation 0-14 days versus 15 days, 0-29 days versus ≥ 30 days
|
Factor |
Interval 0-14 days vs. ≥ 15 days
|
Interval 0-29 days vs. ≥ 30 days
|
|
HR (95% CI) |
p-value |
HR (95% CI) |
p-value |
|
Age (yr)
|
|
|
|
|
|
< 40 vs. ≥ 40 |
1.395 (0.959-2.031) |
0.082 |
1.381 (0.948-2.011) |
0.093 |
|
Tumor size (cm)
|
|
|
|
|
|
> 2 vs. ≤ 2 |
2.176 (1.516-3.124) |
< 0.001 |
2.181 (1.520-3.130) |
< 0.001 |
|
Axillary lymph node metastasis
|
|
|
|
|
|
Positive vs. negative |
2.358 (1.698-3.275) |
< 0.001 |
2.357 (1.698-3.273) |
< 0.001 |
|
Histologic grade
|
|
|
|
|
|
Grade 3 vs. 1, 2 |
1.810 (1.212-2.702) |
0.004 |
1.814 (1.215-2.710) |
0.004 |
|
Hormone receptor
|
|
|
|
|
|
Negative vs. positive |
1.798 (1.262-2.562) |
0.001 |
1.786 (1.253-2.546) |
0.001 |
|
Treatment delay
|
|
|
|
|
|
Shorter vs. longer |
1.145 (0.808-1.622) |
0.448 |
1.109 (0.782-1.572) |
0.561 |

Subgroup analyses were performed to determine which patients with an interval of 30 days and over might significantly have worse DFS. However, no significant association was found for hormone receptor–positive vs. –negative tumor groups, triple-negative breast cancer, younger (< 40 years) vs. older women, clinical stage T2 or greater vs. stage T1 and clinically lymph node–positive vs. –negative groups.
Go to :

Discussion
This study showed that a delay of treatment initiation at any cut-off point within 60 days after biopsy confirmation had no impact on DFS and OS in breast cancer. Although with shorter interval, these results are consistent with the recent study by Brazda et al. [
1], which showed that delays in time to treatment over 90 days had no effect on OS in breast cancer. Mujar et al. [
3] also reported that delays in time to primary treatment over 2 months have no impact on breast cancer survival. However, two population-based cohort studies from Korea reported opposing results and suggested that longer intervals between diagnosis and treatment initiation are related to worse OS in breast cancer [
4,
6]. Both studies used nation-wide cancer registry data as their source for the cancer diagnosis date, and health insurance data for treatment information. Nation-wide databases such as these tend to be limited in the accuracy and detail of their data. In contrast, in our study we used electronic medical record data derived from a single institution, which would be expected to be more accurate and includes detailed clinicopathologic information and cancer recurrence data. We also excluded patients with an unusually long delay of treatment initiation of more than 6 months. Such patients may have ignored their diagnosis of breast cancer, refused standard treatment, or looked for alternative medical treatments.
Previous studies have reported on the impact of treatment delay on patient survival in various subgroups. Smith et al. [
5] found that when younger (< 40 years) patients underwent surgery as their initial treatment, women with a delay in surgical treatment of over 6 weeks had 10% decreased OS compared to women with a delay in surgical treatment of 2 weeks or shorter. McLaughlin et al. [
2] reported that late stage breast cancer patients, including metastatic breast cancer, had a worse survival when treatment delay was 60 days or over, whereas Eastman et al. [
8] found no relationship between treatment delay and OS in triple negative breast cancer. Regarding our study, subgroup analysis showed that age, clinical stage and hormone receptor status had no impact on DFS.
The mean treatment delay of 23 days in our study was slightly shorter compared to reports from western countries (22-46 days) [
1,
2,
5,
8]. This reflects the Korean healthcare system, where fee-for-service reimbursement does not influence treatment delay [
4]. Patients can freely choose their medical attendant and hospital, and delay due to referral is relatively short. On the other hand, this interval is longer than results from a Korean nationwide database (14 days) [
4] due to the fact that SNUH is a tertiary referral center with most patients being diagnosed at other hospitals (92.3%).
Women with comorbidities had significantly longer treatment delay, as these patients required more clinical work up before beginning treatment. In addition, performing procedures or consultation before treatment initiation was associated with treatment delay. Another factor contributing to longer treatment delay was when the patient was diagnosed at other hospitals. Many patients are referred from secondary or tertiary hospitals to central high-volume hospitals in Korea. This increases the patient’s travel distance and can lead to treatment delay [
9-
11], and to over-loading [
12-
14] and provider-related delay in high-volume hospitals. Longer interval in high-volume hospitals was also demonstrated in the report from the Korean Central Cancer Registry [
6].
The retrospective analysis is the limitation of this study. The reason for treatment delay could not be accurately evaluated in this retrospective study. In addition, it was impossible to know the time interval between symptom presentation to diagnosis. Patients who received neoadjuvant chemotherapy were excluded, so that patients with more aggressive tumors might have been excluded resulting in a selection bias. After subgrouping, number of patients in each subgroup was sometimes too small to perform survival analyses, which was another limitation.
Go to :

Conclusion
In conclusion, breast cancer patients who were diagnosed at another hospital or had medical comorbidities were more likely to have a longer interval between diagnosis and treatment initiation. Also, undergoing additional procedures before admission for surgery influenced treatment delay. However treatment delay had no impact on DFS, allowing breast cancer patients to endure the nervous wait until treatment initiation without concern for disease progression.
Go to :

Notes
Go to :

ACKNOWLEDGMENTS
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C3405, HI14C1277, HI13C2148).
Go to :

References
1. Brazda A, Estroff J, Euhus D, Leitch AM, Huth J, Andrews V, et al. Delays in time to treatment and survival impact in breast cancer. Ann Surg Oncol. 2010; 17 Suppl 3:291–6.

2. McLaughlin JM, Anderson RT, Ferketich AK, Seiber EE, Balkrishnan R, Paskett ED. Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J Clin Oncol. 2012; 30:4493–500.

3. Mujar M, Dahlui M, Yip CH, Taib NA. Delays in time to primary treatment after a diagnosis of breast cancer: does it impact survival? Prev Med. 2013; 56:222–4.

4. Shin DW, Cho J, Kim SY, Guallar E, Hwang SS, Cho B, et al. Delay to curative surgery greater than 12 weeks is associated with increased mortality in patients with colorectal and breast cancer but not lung or thyroid cancer. Ann Surg Oncol. 2013; 20:2468–76.

5. Smith EC, Ziogas A, Anton-Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg. 2013; 148:516–23.

6. Yun YH, Kim YA, Min YH, Park S, Won YJ, Kim DY, et al. The influence of hospital volume and surgical treatment delay on long-term survival after cancer surgery. Ann Oncol. 2012; 23:2731–7.

7. Ganz PA. Quality of life across the continuum of breast cancer care. Breast J. 2000; 6:324–30.

8. Eastman A, Tammaro Y, Moldrem A, Andrews V, Huth J, Euhus D, et al. Outcomes of delays in time to treatment in triple negative breast cancer. Ann Surg Oncol. 2013; 20:1880–5.

9. Birkmeyer JD, Siewers AE, Marth NJ, Goodman DC. Regionalization of high-risk surgery and implications for patient travel times. JAMA. 2003; 290:2703–8.

10. Simunovic M, Theriault ME, Paszat L, Coates A, Whelan T, Holowaty E, et al. Using administrative databases to measure waiting times for patients undergoing major cancer surgery in Ontario, 1993-2000. Can J Surg. 2005; 48:137–42.
11. Stitzenberg KB, Sigurdson ER, Egleston BL, Starkey RB, Meropol NJ. Centralization of cancer surgery: implications for patient access to optimal care. J Clin Oncol. 2009; 27:4671–8.

12. Bardell T, Belliveau P, Kong W, Mackillop WJ. Waiting times for cancer surgery in Ontario: 1984-2000. Clin Oncol (R Coll Radiol). 2006; 18:401–9.

13. Bilimoria KY, Ko CY, Tomlinson JS, Stewart AK, Talamonti MS, Hynes DL, et al. Wait times for cancer surgery in the United States: trends and predictors of delays. Ann Surg. 2011; 253:779–85.
14. Kawakami J, Hopman WM, Smith-Tryon R, Siemens DR. Measurement of surgical wait times in a universal health care system. Can Urol Assoc J. 2008; 2:597–603.

Go to :






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download