1. Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977; 197:461–3.

2. Rozencweig M, Hofmann V, Sanders C, Rombaut W, Fruh U, Martz G. In vitro growth of human malignancies in a cloning assay. Recent Results Cancer Res. 1984; 94:1–7.

3. Yamaue H, Tanimura H, Tsunoda T, Tani M, Iwahashi M, Noguchi K, et al. Chemosensitivity testing with highly purified fresh human tumour cells with the MTT colorimetric assay. Eur J Cancer. 1991; 27:1258–63.

4. Vescio RA, Redfern CH, Nelson TJ, Ugoretz S, Stern PH, Hoffman RM. In vivo-like drug responses of human tumors growing in three-dimensional gel-supported primary culture. Proc Natl Acad Sci U S A. 1987; 84:5029–33.

5. Kang SM, Park MS, Chang J, Kim SK, Kim H, Shin DH, et al. A feasibility study of adenosine triphosphate-based chemotherapy response assay (ATP-CRA) as a chemosensitivity test for lung cancer. Cancer Res Treat. 2005; 37:223–7.

6. Cree IA, Kurbacher CM, Untch M, Sutherland LA, Hunter EM, Subedi AM, et al. Correlation of the clinical response to chemotherapy in breast cancer with ex vivo chemosensitivity. Anticancer Drugs. 1996; 7:630–5.

7. Konecny G, Crohns C, Pegram M, Felber M, Lude S, Kurbacher C, et al. Correlation of drug response with the ATP tumorchemosensitivity assay in primary FIGO stage III ovarian cancer. Gynecol Oncol. 2000; 77:258–63.

8. Huh JW, Park YA, Lee KY, Sohn SK. Heterogeneity of adenosine triphosphate-based chemotherapy response assay in colorectal cancer: secondary publication. Yonsei Med J. 2009; 50:697–703.
9. Hur H, Kim NK, Kim HG, Min BS, Lee KY, Shin SJ, et al. Adenosine triphosphate-based chemotherapy response assayguided chemotherapy in unresectable colorectal liver metastasis. Br J Cancer. 2012; 106:53–60.

10. Park S, Woo Y, Kim H, Lee YC, Choi S, Hyung WJ, et al. In vitro adenosine triphosphate based chemotherapy response assay in gastric cancer. J Gastric Cancer. 2010; 10:155–61.
11. Benson AB 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004; 22:3408–19.

12. Schippinger W, Samonigg H, Schaberl-Moser R, Greil R, Thodtmann R, Tschmelitsch J, et al. A prospective randomised phase III trial of adjuvant chemotherapy with 5-fluorouracil and leucovorin in patients with stage II colon cancer. Br J Cancer. 2007; 97:1021–7.

13. Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000; 124:979–94.
14. Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009; 27:3109–16.
15. Quasar Collaborative Group, Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007; 370:2020–9.
16. Gill S, Loprinzi CL, Sargent DJ, Thome SD, Alberts SR, Haller DG, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004; 22:1797–806.

17. Markowitz SD, Bertagnolli MM. Molecular origins of cancer:molecular basis of colorectal cancer. N Engl J Med. 2009; 361:2449–60.
18. Bertagnolli MM, Redston M, Compton CC, Niedzwiecki D, Mayer RJ, Goldberg RM, et al. Microsatellite instability and loss of heterozygosity at chromosomal location 18q: prospective evaluation of biomarkers for stages II and III colon cancer:a study of CALGB 9581 and 89803. J Clin Oncol. 2011; 29:3153–62.

19. Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010; 28:466–74.

20. Salazar R, Roepman P, Capella G, Moreno V, Simon I, Dreezen C, et al. Gene expression signature to improve prognosis prediction of stage II and III colorectal cancer. J Clin Oncol. 2011; 29:17–24.

21. Maak M, Simon I, Nitsche U, Roepman P, Snel M, Glas AM, et al. Independent validation of a prognostic genomic signature (ColoPrint) for patients with stage II colon cancer. Ann Surg. 2013; 257:1053–8.

22. Kim HA, Yom CK, Moon BI, Choe KJ, Sung SH, Han WS, et al. The use of an in vitro adenosine triphosphate-based chemotherapy response assay to predict chemotherapeutic response in breast cancer. Breast. 2008; 17:19–26.

23. Lee JH, Kim MC, Oh SY, Kwon HC, Kim SH, Kwon KA, et al. Predictive value of in vitro adenosine triphosphate-based chemotherapy response assay in advanced gastric cancer patients who received oral 5-Fluorouracil after curative resection. Cancer Res Treat. 2011; 43:117–23.

24. Meyers M, Wagner MW, Hwang HS, Kinsella TJ, Boothman DA. Role of the hMLH1 DNA mismatch repair protein in fluoropyrimidine-mediated cell death and cell cycle responses. Cancer Res. 2001; 61:5193–201.
25. Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003; 349:247–57.

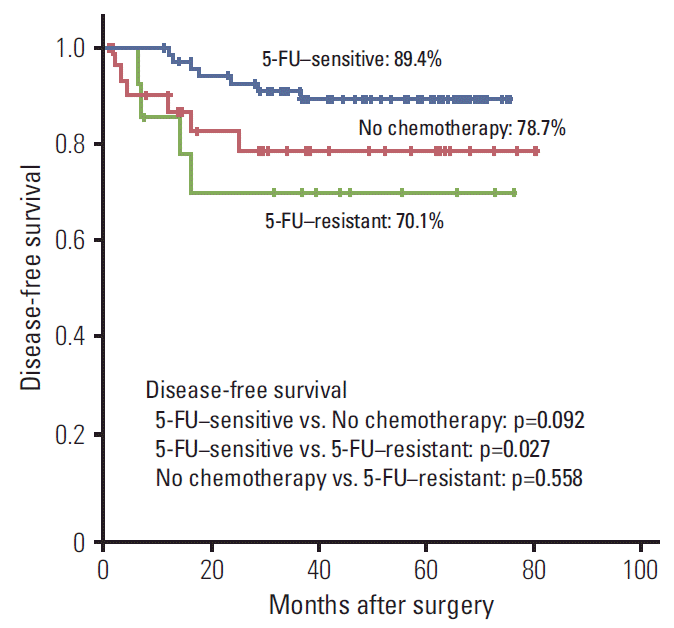
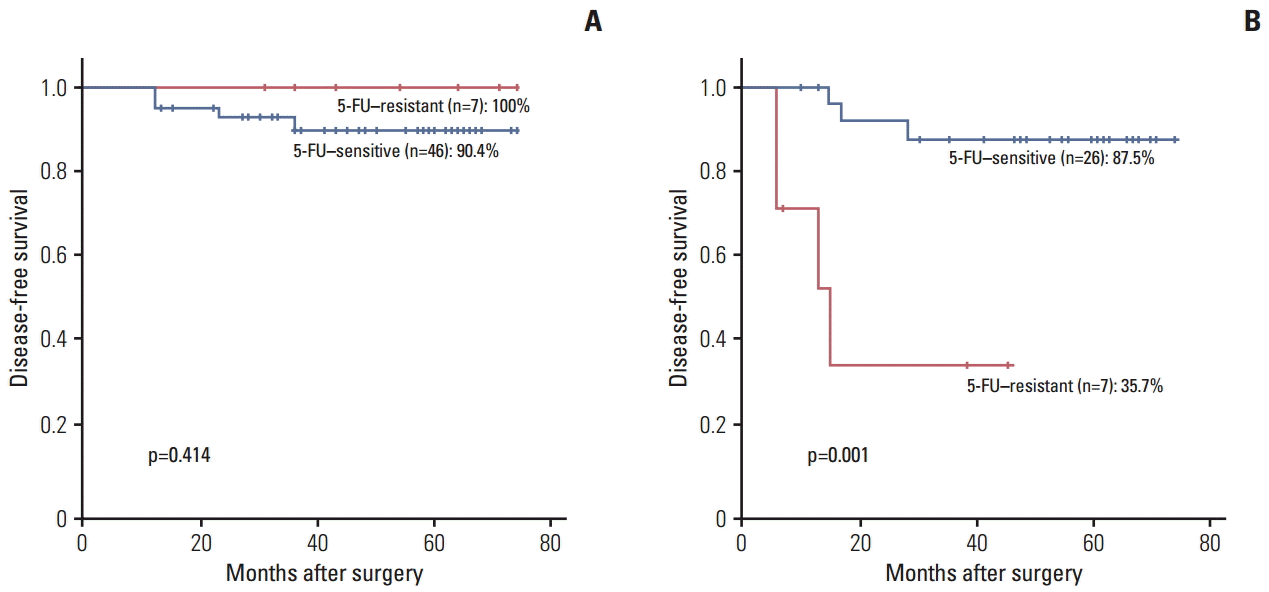




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download