Abstract
Purpose
Many end-of-life care studies are based on the assumption that there is a shared definition of language concerning the stage of cancer. However, studies suggest that patients and their families often misperceive patients’ cancer stages and prognoses. Discrimination between advanced cancer and terminal cancer is important because the treatment goals are different. In this study, we evaluated the understanding of the definition of advanced versus terminal cancer of the general population and determined associated socio-demographic factors.
Materials and Methods
A total of 2,000 persons from the general population were systematically recruited. We used a clinical vignette of a hypothetical advanced breast cancer patient, but whose cancer was not considered terminal. After presenting the brief history of the case, we asked respondents to choose the correct cancer stage from a choice of early, advanced, terminal stage, and don’t know. Multinomial logistic regression analysis was performed to determine sociodemographic factors associated with the correct response, as defined in terms of medical context.
Results
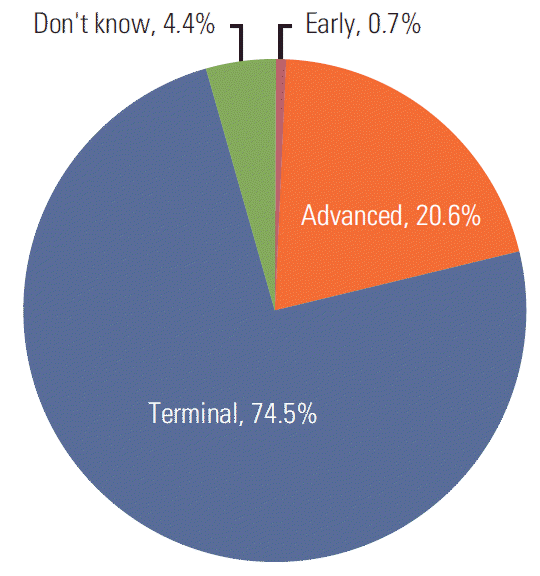
Only 411 respondents (20.6%) chose “advanced,” while most respondents (74.5%) chose “terminal stage” as the stage of the hypothetical patient, and a small proportion of respondents chose “early stage” (0.7%) or “don’t know” (4.4%). Multinomial logistic regression analysis found no consistent or strong predictor.
Conclusion
A large proportion of the general population could not differentiate advanced cancer from terminal cancer. Continuous effort is required in order to establish common and shared definitions of the different cancer stages and to increase understanding of cancer staging for the general population.
A shared understanding of a patient’s status between physicians, the patient, and caregivers is important in shared decision-making. Not only can it reduce the discrepancy in expectations regarding treatment objectives, it can also enable more appropriate decision-making for patients [1]. Precise awareness of disease status, i.e., shared understanding of cancer stages between cancer patients and their caregivers, is essential for shared decision-making.
Discrimination between advanced and terminal cancer is important because the treatment goals are different. In cases of advanced cancer, which is not curable but responds to treatment, disease-directed therapy is still very important because it prolongs life. However, for terminal cancer, therapy cannot prolong survival significantly due to the progressive nature of the disease [2-5]. Therefore, palliative care that provides psychosocial support and controls symptoms is the main treatment option for patients with terminal cancer [6,7]. Accurate prognostic understanding may affect decision-making towards more appropriate care [8]. However, studies suggest that patients and their families often misperceive the patient’s cancer stage and prognosis [9-11], leading to unrealistic expectations regarding the treatment effect and inappropriate treatment decisions [9,12-14].
In addition, many end-of-life care studies are based on the assumption that there is a shared definition of language regarding the cancer stage among patients, caregivers, the general population, and physicians [15]. For example, Yun et al. [16,17] assessed patients’ awareness of terminal illness soon after they were considered terminal by their doctor with the following question: “Do you know your disease stage?” Response options included, “I don’t know,” “early stage,” “advanced stage,” “terminal stage,” and “other.” The authors regarded “terminal stage” as the correct awareness of the terminal nature of the illness [16,17]. Without a shared understanding regarding the definition of terminal stage cancer of physicians and respondents, the validity of such research could be compromised.
Therefore, the lack of a common understanding of words used in cancer care is a critical problem in communication within the clinical practice and the research environment [18]. However, few studies evaluating public awareness regarding the definition of cancer stages have been reported. In a study conducted in Korea, Lee et al. [19] surveyed participants regarding the correct definition of terminal cancer. Five choices were given and “6-month life expectancy” and “treatment refractoriness” were regarded as the correct answers. In that study, 29.4% of the participants from the general population misunderstood locally advanced cancer and metastatic/recurrent disease as terminal stage disease. However, this study was limited in that the response options were not mutually exclusive, and the authors asked for a conceptual definition rather than using an actual situation [19].
In this study, a survey was conducted to evaluate the understanding of the definition of advanced versus terminal cancer in the general population and determined associated socio-demographic factors.
In 2012, the survey examined members of the general population to explore their views on cancer and cancer care. A nationwide home visiting survey was conducted from November 1, 2012 to December 1, 2012. After stratification by the region, samples were systematically extracted according to the population ratio. Inclusion criteria were as follows: (1) a member of the general population aged 40 years or older and younger than 70 years and (2) a member of the general population who was never diagnosed with any type of cancer. The sampling error within a 95% confidence interval was ±2.2%. This study was approved by the Institutional Review Board of the National Cancer Center.
To assess the understanding of the definition of advanced versus terminal cancer in the general population, we developed a clinical vignette of an advanced breast cancer patient with lung metastases, who is however not considered to have a terminal stage cancer, as defined in terms of medical context. The detailed patient history of the vignette is as follows:
“A hypothetical patient (Mrs. Kim) was diagnosed with breast cancer 4 years ago and received chemotherapy due to lung metastases detected 1 year ago. While chemotherapy was effective during the first round of treatment, the tumor subsequently continued to grow. Her doctor said that complete remission was impossible, but prolonged survival could be achieved by tumor size reduction using chemotherapy combined with another regimen.”
After presenting the hypothetical case of Mrs. Kim to participants, we asked about her present cancer stage. Respondents could choose one of the following stages: (1) early, (2) advanced, (3) terminal stage, and (4) don’t know.
We also asked respondents to provide their socio-demographic information.
Multinomial logistic regression was performed to determine related factors for respondents’ answers. For selection of covariates for adjustment in the multinomial logistic regression model, univariate analysis was performed for each of the socio-demographic characteristics and answered stages. Characteristics with p-values less than 0.1 were included in the multinomial logistic regression model. All statistical analyses were performed using the STATA software ver. 13.0 (StataCorp., College Station, TX); p-values less than 0.05 were considered statistically significant.
A total of 2,000 persons (response rate, 41.2%) responded to the survey questions. The socio-demographic characteristics of the respondents are summarized in Table 1.
The mean age was 51.7 years and 991 respondents (49.6%) were men. Most of the participants (94.4%) were married. In terms of education, 1,677 respondents (70.2%) graduated high school or above and 1,672 respondents (83.6%) earned more than 2 million Korean Won per month. Eight hundred thirty-nine participants (42.0%) had relatives or acquaintances diagnosed with cancer.
As shown in Fig. 1, only 411 respondents (20.6%) chose “advanced stage.” Surprisingly, most respondents chose “terminal stage” as the stage of the hypothetical case (n=1,489, 74.5%). A small proportion of respondents chose “early stage” (n=13, 0.7%) or “don’t know” (n=87, 4.4%).
In univariate analysis, marital status (p=0.019), educational status (p < 0.001), and income (p=0.021) significantly affected the answer concerning the cancer stage of the hypothetical case. Therefore, marital status, educational status, and income status were included as covariates in multivariate analysis. As the p-values of sex and 'Cancer patients in relatives or acquaintances' were less than 0.1 in univariate analysis, they were also included as covariates (Table 2). Multivariate multinomial logistic regression analyses were performed. Respondents who chose “advanced stage” were included in the reference group. Male respondents were less likely to answer “early stage” (odds ratio [OR], 0.15; p=0.016). Respondents who did not have relatives or acquaintances with cancer chose “terminal stage” less frequently than those who had relatives or acquaintances with cancer (OR, 0.77; p=0.022). Respondents with educational status of high school (OR, 0.40; p=0.005) or above (OR, 0.22; p < 0.001) answered “don’t know” significantly less frequently than those with educational status below high school. However, there were no consistent and strong predictors across the choices (Table 3). The same analysis was also performed with the respondents who chose “terminal stage” as the reference group. The results were almost the same as those of the main analysis. Male respondents were more likely to answer “terminal stage” than “early stage” (OR, 0.15; p=0.015). In addition, respondents who had relatives or acquaintances with cancer chose “advanced stage” more frequently than those who had no relatives or acquaintances with cancer (OR, 1.30; p=0.022) (Table 4).
To the best of our knowledge, this is the first study assessing the understanding of the definition of advanced versus terminal cancer in the general population using a hypothetical vignette about a case of advanced cancer. We found that a large proportion (74.5%) of the general population misunderstood the medical definition of terminal cancer, and could not discriminate terminal cancer from advanced cancer with metastasis.
The proportion of inadequate responses was as high as 80%, even higher than that reported in previous survey results (one-third of participants answered incorrectly) on the public perception of the definition of terminal cancer in South Korea. Although there are several exceptions, many patients with advanced stage cancers cannot expect to be cured, and have a poor long-term prognosis. Despite the availability of life-prolonging treatment, most patients will eventually die because of their cancers. The results of our survey suggest that most members of the general population seem to not be familiar with the concept of ”treatment refractoriness” and “life expectancy less than 6 months,” and may regard advanced stage cancer as terminal cancer [19]. Differences in the correct response rates across different studies can be explained by differences in study design. In a previous study, in which five response options for the definition of terminal cancer were provided to a conceptual question, many respondents could have chosen the correct option by chance or by avoiding extreme options (“a cancer still resectable and curable” or “a cancer with a prognosis of death within a few days or weeks”). Therefore, a continuous effort is required in order to establish common and shared definitions of cancer staging and to increase awareness across the general population [18].
The results of this study have several important clinical and research implications. First, members of the general population can become cancer patients themselves or caregivers of cancer patients in the future. Misunderstanding about the accurate cancer stage can lead to discrepancies in expectation regarding the treatment effect between patients, caregivers, and physicians [20]. Although doctors inform patients of their exact cancer stages, patients and caregivers might not understand the prognostic meaning of the cancer stage. Providing patients and their families with a full understanding of the disease status is very difficult for physicians [21], and physicians rarely check their patient’s understanding of the diagnosis [22]. Therefore, for informed decision-making or shared understanding, it is not sufficient to simply provided information on the stage of the cancer as early, advanced, or terminal. For patients, the perception of terminal cancer can influence decision-making regarding the treatment plan [23]. This misunderstanding can lead to an undesirable scenario, in which patients forgo treatment when diagnosed with advanced cancer that could prolong survival. For patients with terminal cancers, this misunderstanding may lead to precious time being occupied with futile disease-directed treatment options [24]. When shared understanding about the stage of the cancer is lacking, the decision-making process cannot guarantee an appropriate outcome. Therefore, for informed decision-making, it is essential for physicians to not only provide information on the exact cancer stage, but to also explain the meaning of that stage and to confirm the patient’s understanding [25].
Second, there was no consistent and strong predictor as to which respondents would answer correctly, as defined in terms of medical context. However, some factors were partially associated with the choices. Interestingly, the educational status of the participants was not significantly associated with choosing the correct answer. In addition, personal experience with relatives or acquaintances diagnosed with cancer also did not improve the understanding of cancer staging, and even increased the frequency with which respondents chose the response option of “terminal cancer.“ The experience of cancer in close relationships could affect the perceived knowledge of the general population, resulting in a more sensitive reaction than those who had no relatives or acquaintances with cancer. Thus, they chose “terminal stage” more frequently in this study. Although no significant difference in sex was observed between the response groups “advanced stage” and “terminal stage,” female tended to choose “early stage” more frequently. As the hypothetical case in our vignette was a breast cancer patient, females might react with more emotion. However the result is limited to those who chose “early stage” (n=13). In addition, marital status and respondents’ income were not consistently associated with their choices. These results suggest that physicians should verify the understanding of cancer stages during communication with cancer patients and their caregivers regardless of their socio-demographic characteristics and personal experiences.
Finally, many studies based on survey data of the general population regarding terminal disease relied on the correct perception of “terminal cancer.” Some of the surveys did not inform participants of the exact definition of terminal disease, which would be sufficient to ensure that patients have the same understanding as the researchers [15]. However, considering the high proportion of the general population lacking accurate knowledge of the medical definition of terminal cancer, the reliability and validity of those surveys might be compromised. For example, in surveys on topics such as the recently issued legalization of euthanasia, accurate understanding of terminal disease is required for the response to be valid. Therefore, our study suggests that more care should be taken to ensure that respondents of such surveys have a shared understanding of the exact definition of advanced or terminal disease.
The strengths of this study include systematic sampling methods and the large sample size, ensuring the generalizability of these findings across the Korean population. Another advantage of this study is the use of a clinical vignette. Clinical vignettes do not require in-depth knowledge of the study topics. Therefore, the practical understanding of the respondents can be better assessed using clinical vignettes based on a hypothetical case than by using questions based on conceptual knowledge.
There are some limitations in our study.
One notable limitation is that it included only members of the general population. Many of the respondents had not experienced advanced or terminal cancer, and these participants may have less knowledge about cancer than those affected by cancer. However, the proportions of the correct answer between the general population and patients or family caregivers were not significantly different in a previous study [19]. In addition, personal experiences with cancer did not significantly affect the response rate in our study, suggesting that a potential bias would not be significant. Moreover members of the general population can become patients themselves and their close caregivers or friends may influence medical decisions. Therefore, understanding of cancer staging of the general population is relevant from a clinical and research perspective. Development of a communication strategy for end-of-life discussions would also be important.
Second, the staging terms for cancer are defined based on the viewpoint of medicine. Thus, most of the general population might not perceive the exact definitions of the terms for cancer staging. However, as mentioned above, clinical vignettes do not require in-depth knowledge of the study topics. Therefore the results of our study might reflect the understanding of the definition of cancer stages in the naive general population. However, if we asked about the possibility of complete cure, necessity for aggressive chemotherapy for life prolongation, or expected survival time of the hypothetical patient in the vignette, more of the general population might have answered correctly, as defined in terms of medical context. Also, a well-designed study comparing before and after adequate education using patient decision aids regarding the definition of the cancer stages may be needed for more sensitive evaluation of the knowledge in the general population.
In conclusion, we found that a large proportion of the general population could not differentiate advanced cancer from terminal cancer, as defined in terms of medical context. Continuous effort is required in order to establish common and shared definitions of cancer stages and to increase the shared understanding of cancer staging for patients, caregivers, and the general population. In addition, a detailed explanation concerning the stage of the cancer and its clinical meaning is essential for shared understanding and informed decision-making between cancer patients and their families and physicians. Researchers need to ensure that respondents to surveys and studies have a shared understanding of the definition of advanced or terminal disease.
ACKNOWLEDGMENTS
This work was supported by a grant from the National R&D Program for Cancer Control, No. 1210150.
References
1. Frosch DL, Kaplan RM. Shared decision making in clinical medicine: past research and future directions. Am J Prev Med. 1999; 17:285–94.

2. McCusker J. The terminal period of cancer: definition and descriptive epidemiology. J Chronic Dis. 1984; 37:377–85.

3. American Cancer Society. Advance directives: why do you need an advance directive? [Internet]. Mobile, AL: American Cancer Society;2015. [cited 2015 Aug 1]. Available from: http://www.cancer.org/treatment/findingandpayingfortreatment/understandingfinancialandlegalmatters/advancedirectives/advance-directives-why-do-we-need-advance-directives.
4. Christakis NA, Escarce JJ. Survival of medicare patients after enrollment in hospice programs. N Engl J Med. 1996; 335:172–8.

5. Llobera J, Esteva M, Rifa J, Benito E, Terrasa J, Rojas C, et al. Terminal cancer. duration and prediction of survival time. Eur J Cancer. 2000; 36:2036–43.
6. Levy MH, Back A, Benedetti C, Billings JA, Block S, Boston B, et al. NCCN clinical practice guidelines in oncology: palliative care. J Natl Compr Canc Netw. 2009; 7:436–73.
7. Swetz KM, Smith TJ. Palliative chemotherapy: when is it worth it and when is it not? Cancer J. 2010; 16:467–72.
8. Temel JS, Greer JA, Admane S, Gallagher ER, Jackson VA, Lynch TJ, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol. 2011; 29:2319–26.

9. Oh DY, Kim JE, Lee CH, Lim JS, Jung KH, Heo DS, et al. Discrepancies among patients, family members, and physicians in Korea in terms of values regarding the withholding of treatment from patients with terminal malignancies. Cancer. 2004; 100:1961–6.

10. Hagerty RG, Butow PN, Ellis PM, Dimitry S, Tattersall MH. Communicating prognosis in cancer care: a systematic review of the literature. Ann Oncol. 2005; 16:1005–53.

11. Shim HY, Park JH, Kim SY, Shin DW, Shin JY, Park BY, et al. Discordance between perceived and actual cancer stage among cancer patients in Korea: a nationwide survey. PLoS One. 2014; 9:e90483.

12. Weeks JC, Catalano PJ, Cronin A, Finkelman MD, Mack JW, Keating NL, et al. Patients' expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012; 367:1616–25.

13. Lux MP, Bayer CM, Loehberg CR, Fasching PA, Schrauder MG, Bani MR, et al. Shared decision-making in metastatic breast cancer: discrepancy between the expected prolongation of life and treatment efficacy between patients and physicians, and influencing factors. Breast Cancer Res Treat. 2013; 139:429–40.

14. Chow E, Andersson L, Wong R, Vachon M, Hruby G, Franssen E, et al. Patients with advanced cancer: a survey of the understanding of their illness and expectations from palliative radiotherapy for symptomatic metastases. Clin Oncol (R Coll Radiol). 2001; 13:204–8.

15. Yun YH, Han KH, Park S, Park BW, Cho CH, Kim S, et al. Attitudes of cancer patients, family caregivers, oncologists and members of the general public toward critical interventions at the end of life of terminally ill patients. CMAJ. 2011; 183:E673–9.

16. Yun YH, Kwon YC, Lee MK, Lee WJ, Jung KH, Do YR, et al. Experiences and attitudes of patients with terminal cancer and their family caregivers toward the disclosure of terminal illness. J Clin Oncol. 2010; 28:1950–7.

17. Yun YH, Lee MK, Kim SY, Lee WJ, Jung KH, Do YR, et al. Impact of awareness of terminal illness and use of palliative care or intensive care unit on the survival of terminally ill patients with cancer: prospective cohort study. J Clin Oncol. 2011; 29:2474–80.
18. Coalition to Transform Advanced Care. Public perceptions of advanced illness care: how can we talk when there’s no shared language? [Internet]. Washington, DC: Coalition to Transform Advanced Care;2012. [cited 2015 Aug 1]. Available from: http://advancedcarecoalition.org/wp-content/uploads/2011/10/Consumer-Research-Brief2.pdf.
19. Lee JK, Yun YH, An AR, Heo DS, Park BW, Cho CH, et al. The understanding of terminal cancer and its relationship with attitudes toward end-of-life care issues. Med Decis Making. 2013; 34:720–30.

20. Mackillop WJ, Stewart WE, Ginsburg AD, Stewart SS. Cancer patients' perceptions of their disease and its treatment. Br J Cancer. 1988; 58:355–8.

21. Hancock K, Clayton JM, Parker SM, Walder S, Butow PN, Carrick S, et al. Discrepant perceptions about end-of-life communication: a systematic review. J Pain Symptom Manage. 2007; 34:190–200.

22. Perkins HS. The varied understandings of "terminal cancer" and their implications for clinical research and patient care. Med Decis Making. 2014; 34:696–8.

23. Ahn E, Shin DW, Choi JY, Kang J, Kim DK, Kim H, et al. The impact of awareness of terminal illness on quality of death and care decision making: a prospective nationwide survey of bereaved family members of advanced cancer patients. Psychooncology. 2013; 22:2771–8.

Table 1.
Baseline characteristics of the respondents
Table 2.
Answered stage according to respondent characteristics
| Characteristic |
Answered stage |
p-valuea) | ||||
|---|---|---|---|---|---|---|
| Early | Advanced | Terminal | Don't know | Total | ||
| Age (yr) | ||||||
| Elderly (≥ 65) | 1 (0.9) | 22 (19.0) | 83 (71.6) | 10 (8.6) | 116 | 0.127 |
| Young (< 65) | 12 (0.6) | 389 (20.6) | 1,406 (74.6) | 77 (4.1) | 1,884 | |
| Sex | ||||||
| Male | 2 (0.2) | 207 (20.9) | 743 (75.0) | 39 (3.9) | 991 | 0.067 |
| Female | 11 (1.1) | 204 (20.2) | 746 (73.9) | 48 (4.8) | 1,009 | |
| Marital status | ||||||
| Married | 13 (0.7) | 387 (20.5) | 1,413 (74.8) | 75 (4.0) | 1,888 | 0.019 |
| Unmarried | 0 | 24 (21.4) | 76 (67.9) | 12 (10.7) | 112 | |
| Educational status | ||||||
| Less than high school (< 9 yr) | 1 (0.3) | 56 (17.3) | 236 (73.1) | 30 (9.3) | 323 | < 0.001 |
| High school (9-12 yr) | 6 (0.6) | 214 (20.6) | 779 (74.8) | 42 (4.0) | 1,041 | |
| College and above (> 12 yr) | 6 (0.9) | 141 (22.2) | 474 (74.5) | 15 (2.4) | 636 | |
| Income status | ||||||
| < 2 million KRW | 1 (0.9) | 64 (19.5) | 238 (72.6) | 25 (7.6) | 328 | 0.021 |
| ≥ 2 million KRW | 12 (0.7) | 347 (20.8) | 1,251 (74.8) | 62 (3.7) | 1,672 | |
| Smoking | ||||||
| Current smoker | 0 | 124 (20.8) | 447 (74.9) | 26 (4.4) | 597 | 0.267 |
| Past smoker | 4 (1.2) | 65 (19.8) | 246 (74.8) | 14 (4.3) | 329 | |
| Never smoked | 9 (0.8) | 222 (20.7) | 796 (74.1) | 47 (4.4) | 1,074 | |
| Alcohol drinking | ||||||
| Current drinker | 6 (0.4) | 298 (20.7) | 1,080 (75.0) | 56 (3.9) | 1,440 | 0.142 |
| Past drinker | 4 (1.9) | 45 (20.8) | 154 (71.3) | 13 (6.0) | 216 | |
| Never drunk | 3 (0.9) | 68 (19.8) | 255 (74.1) | 18 (5.2) | 344 | |
| Cancer patients in relatives or acquaintances | ||||||
| Present | 5 (0.6) | 151 (18.0) | 641 (76.4) | 42 (5.0) | 839 | 0.075 |
| Absent | 8 (0.7) | 260 (22.4) | 848 (73.0) | 45 (3.9) | 1,161 | |
| Looked after relatives or acquaintances as a care giver | ||||||
| Yes | 3 (0.7) | 92 (20.1) | 337 (73.6) | 26 (5.7) | 458 | 0.449 |
| No | 10 (0.6) | 319 (20.7) | 1,152 (74.7) | 61 (4.0) | 1,542 | |
| Comorbidities | ||||||
| Present | 3 (0.6) | 93 (19.4) | 354 (73.8) | 30 (6.3) | 480 | 0.135 |
| Absent | 10 (0.7) | 318 (20.9) | 1,135 (74.7) | 57 (3.8) | 1,520 | |
| Total | 13 (0.7) | 411 (20.6) | 1,489 (74.5) | 87 (4.4) | 2,000 | |
Table 3.
Multinomial logistic regression (using answered “advanced” as a reference outcome)
| Variable | Odds ratio | p-value |
|---|---|---|
| Answered 'early' (n=13) | ||
| Sex | ||
| Female (reference) | 1.00 | |
| Male | 0.15 | 0.016 |
| Marital status | ||
| Unmarried (reference) | 1.00 | |
| Married | a) | a) |
| Educational status | ||
| Less than high school (< 9 yr) (reference) | 1.00 | |
| High school (9-12 yr) | 1.36 | 0.796 |
| College and above (> 12 yr) | 2.69 | 0.413 |
| Income status | ||
| < 2 million KRW (reference) | 1.00 | |
| ≥ 2 million KRW | 1.29 | 0.826 |
| Cancer patients in relatives or acquaintances | ||
| Present (reference) | 1.00 | |
| Absent | 0.92 | 0.879 |
| Answered 'terminal' (n=1,489) | ||
| Sex | ||
| Female (reference) | 1.00 | |
| Male | 1.00 | 0.966 |
| Marital status | ||
| Unmarried (reference) | 1.00 | |
| Married | 1.19 | 0.480 |
| Educational status | ||
| Less than high school (< 9 yr) (reference) | 1.00 | |
| High school (9-12 yr) | 0.85 | 0.375 |
| College and above (> 12 yr) | 0.77 | 0.195 |
| Income status | ||
| < 2 million KRW (reference) | 1.00 | |
| ≥ 2 million KRW | 1.05 | 0.765 |
| Cancer patients in relatives or acquaintances | ||
| Present (reference) | 1.00 | |
| Absent | 0.77 | 0.022 |
| Answered 'don't know' (n=87) | ||
| Sex | ||
| Female (reference) | 1.00 | |
| Male | 0.97 | 0.912 |
| Marital status | ||
| Unmarried (reference) | 1.00 | |
| Married | 0.51 | 0.091 |
| Educational status | ||
| Less than high school (< 9 yr) (reference) | 1.00 | |
| High school (9-12 yr) | 0.40 | 0.005 |
| College and above (> 12 yr) | 0.22 | < 0.001 |
| Income status | ||
| < 2 million KRW (reference) | 1.00 | |
| ≥ 2 million KRW | 0.97 | 0.931 |
| Cancer patients in relatives or acquaintances | ||
| Present (reference) | 1.00 | |
| Absent | 0.64 | 0.066 |
Table 4.
Multinomial logistic regression (answered terminal as a reference outcome)
| Variable | Odds ratio | p-value |
|---|---|---|
| Answered 'early' (n=13) | ||
| Sex | ||
| Female (reference) | 1.00 | |
| Male | 0.15 | 0.015 |
| Marital status | ||
| Unmarried (reference) | 1.00 | |
| Married | a) | a) |
| Educational status | ||
| Less than high school (< 9 yr) (reference) | 1.00 | |
| High school (9-12 yr) | 1.60 | 0.688 |
| College and above (> 12 yr) | 3.50 | 0.297 |
| Income status | ||
| < 2 million KRW (reference) | 1.00 | |
| ≥ 2 million KRW | 1.22 | 0.861 |
| Cancer patients in relatives or acquaintances | ||
| Present (reference) | 1.00 | |
| Absent | 1.19 | 0.759 |
| Answered 'advanced' (n=411) | ||
| Sex | ||
| Female (reference) | 1.00 | |
| Male | 1.00 | 0.966 |
| Marital status | ||
| Unmarried (reference) | 1.00 | |
| Married | 0.84 | 0.480 |
| Educational status | ||
| Less than high school (< 9 yr) (reference) | 1.00 | |
| High school (9-12 yr) | 1.18 | 0.375 |
| College and above (> 12 yr) | 1.30 | 0.195 |
| Income status | ||
| < 2 million KRW (reference) | 1.00 | |
| ≥ 2 million KRW | 0.95 | 0.765 |
| Cancer patients in relatives or acquaintances | ||
| Present (reference) | 1.00 | |
| Absent | 1.30 | 0.022 |
| Answered 'unknown' (n=87) | ||
| Sex | ||
| Female (reference) | 1.00 | |
| Male | 0.97 | 0.889 |
| Marital status | ||
| Unmarried (reference) | 1.00 | |
| Married | 0.43 | 0.016 |
| Educational status | ||
| Less than high school (< 9 yr) (reference) | 1.00 | |
| High school (9-12 yr) | 0.48 | 0.010 |
| College and above (> 12 yr) | 0.29 | 0.001 |
| Income status | ||
| < 2 million KRW (reference) | 1.00 | |
| ≥ 2 million KRW | 0.92 | 0.786 |
| Cancer patients in relatives or acquaintances | ||
| Present (reference) | 1.00 | |
| Absent | 0.84 | 0.429 |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download