Introduction
Materials and Methods
1. In vivo tumorigenicity
2. Establishment of heterotopic and orthotopic murine tumor models
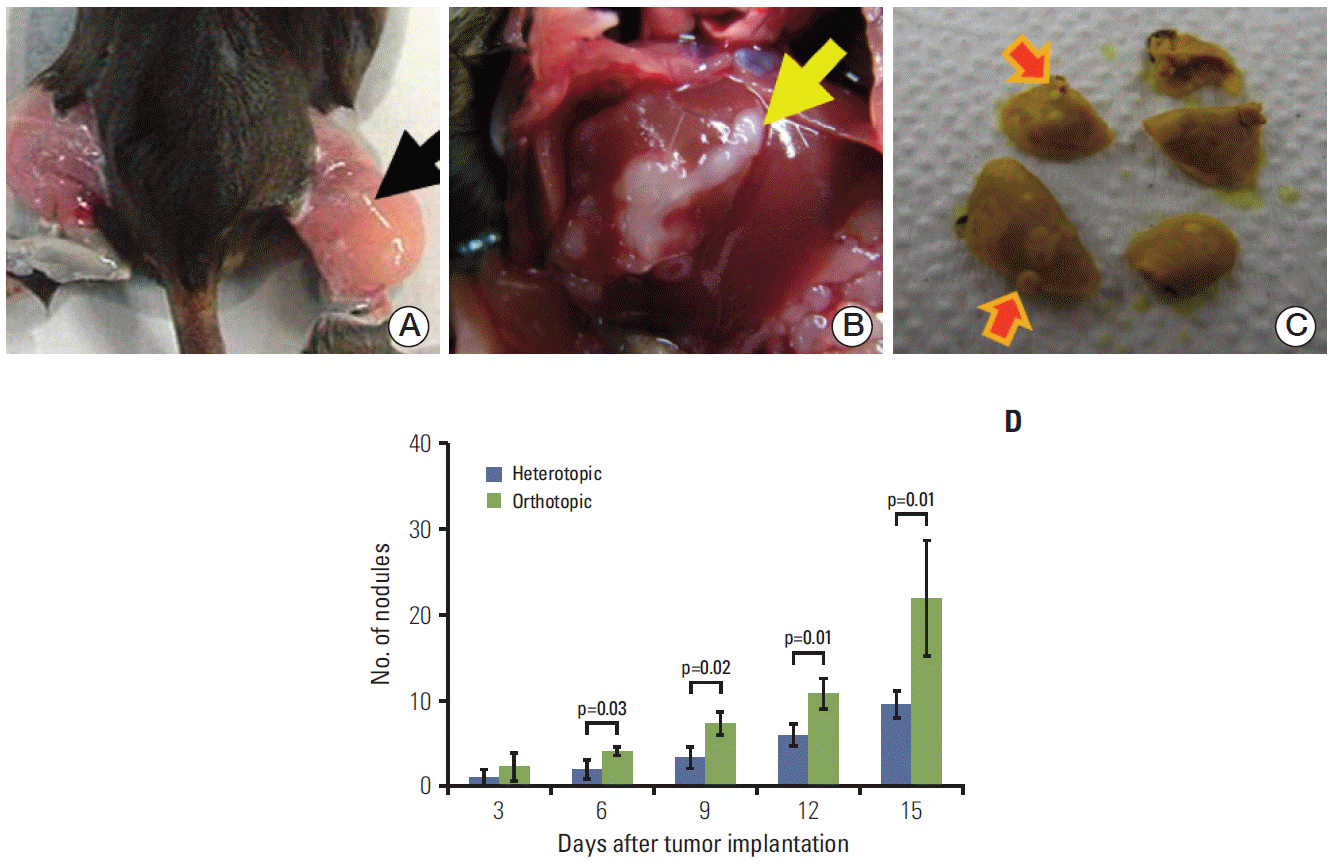 | Fig. 1.Tumor cells were implanted in the thigh muscle (arrow) in the heterotopic tumor model (A) and at a subcapsular site (arrow) in the liver in the orthotopic tumor model (B). The frequency of lung metastases (arrows) was determined after fixation with Bouin’s solution (C) under a light microscope. The number of metastatic lung nodules was higher in the orthotopic tumor model than in the heterotopic tumor model (D). The number of metastatic lung nodules was found to significantly increase in the orthotopic tumor model at 6 (p=0.03), 9 (p=0.02), 12 (p=0.01), and 15 days (p=0.01) after tumor implantation. Groups consisted of six mice each. |
3. Frequency of lung metastases associated with heterotopic versus orthotopic murine tumors
4. Radiation administration to the orthotopic murine tumor
5. Immunohistochemical staining of tumor microenvironmental molecules
6. Western blot analysis of tumor microenvironmental molecules
7. Determination of the host response to different microenvironments by enzyme-linked immunosorbent assay
8. Immune response of the normal peritumor liver and tumor by flow cytometry
9. Statistical analysis
Results
1. Establishment of different TME animal models for murine hepatocarcinoma
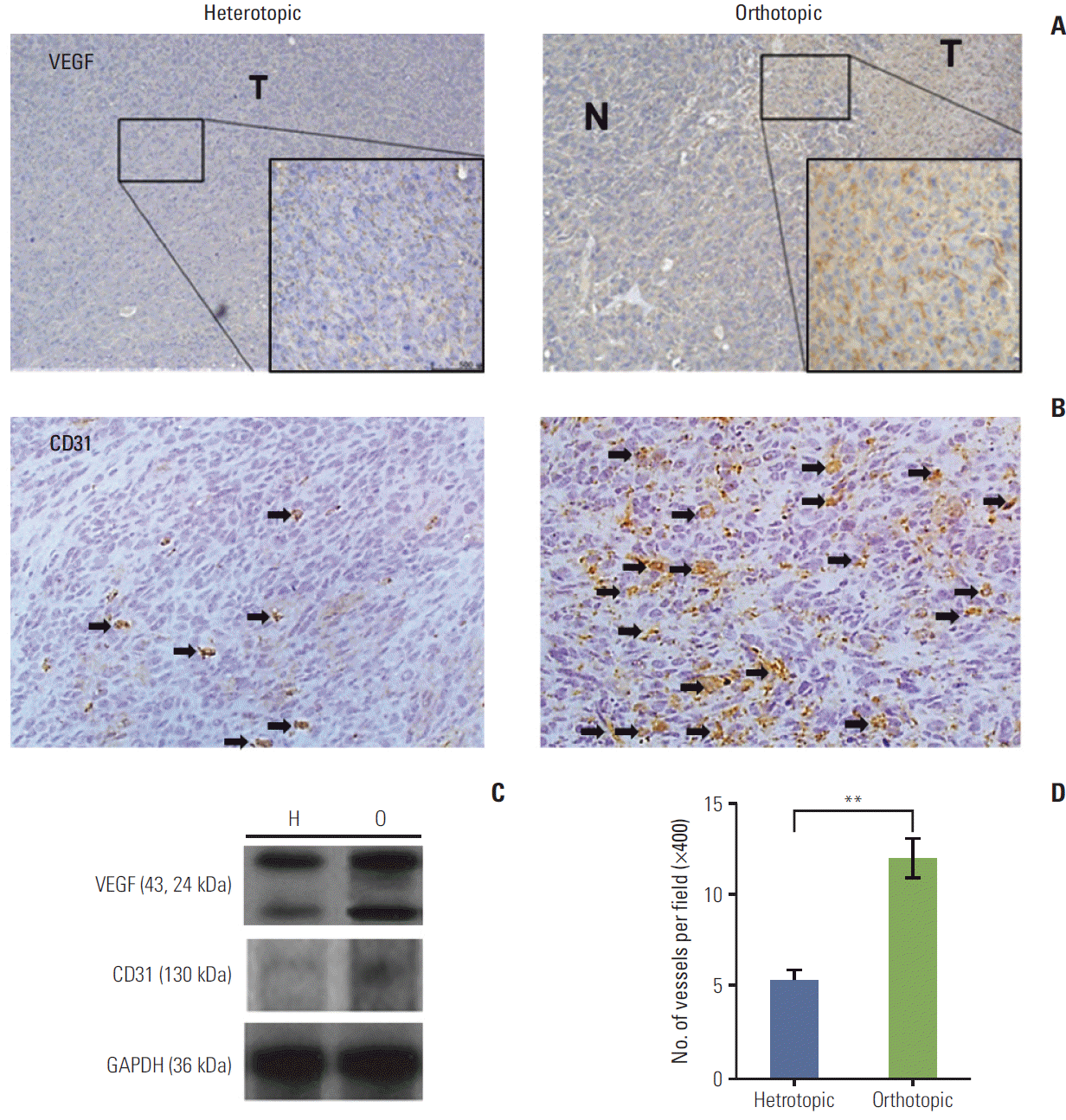 | Fig. 2.(A) The orthotopic tumor model showed intense cytoplasmic immunoreactivity (×400). N, peritumor normal liver; T, tumor tissue. (B) The immunohistochemical stains of CD31 (arrows) for heterotopic and orthotopic tumor model (×400). (C) Vascular endothelial growth factor (VEGF) protein levels were elevated in the orthotopic tumor model (O) compared with the heterotopic tumor model (H). CD31 expression was also higher in the orthotopic model than in the heterotopic tumor model. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. (D) Microvessel density using CD31 increased significantly in the orthotopic tumor model (**p < 0.01). Arrows indicate the microvessels of positive CD31 immunohistochemical staining. The number of mice was six per group. |
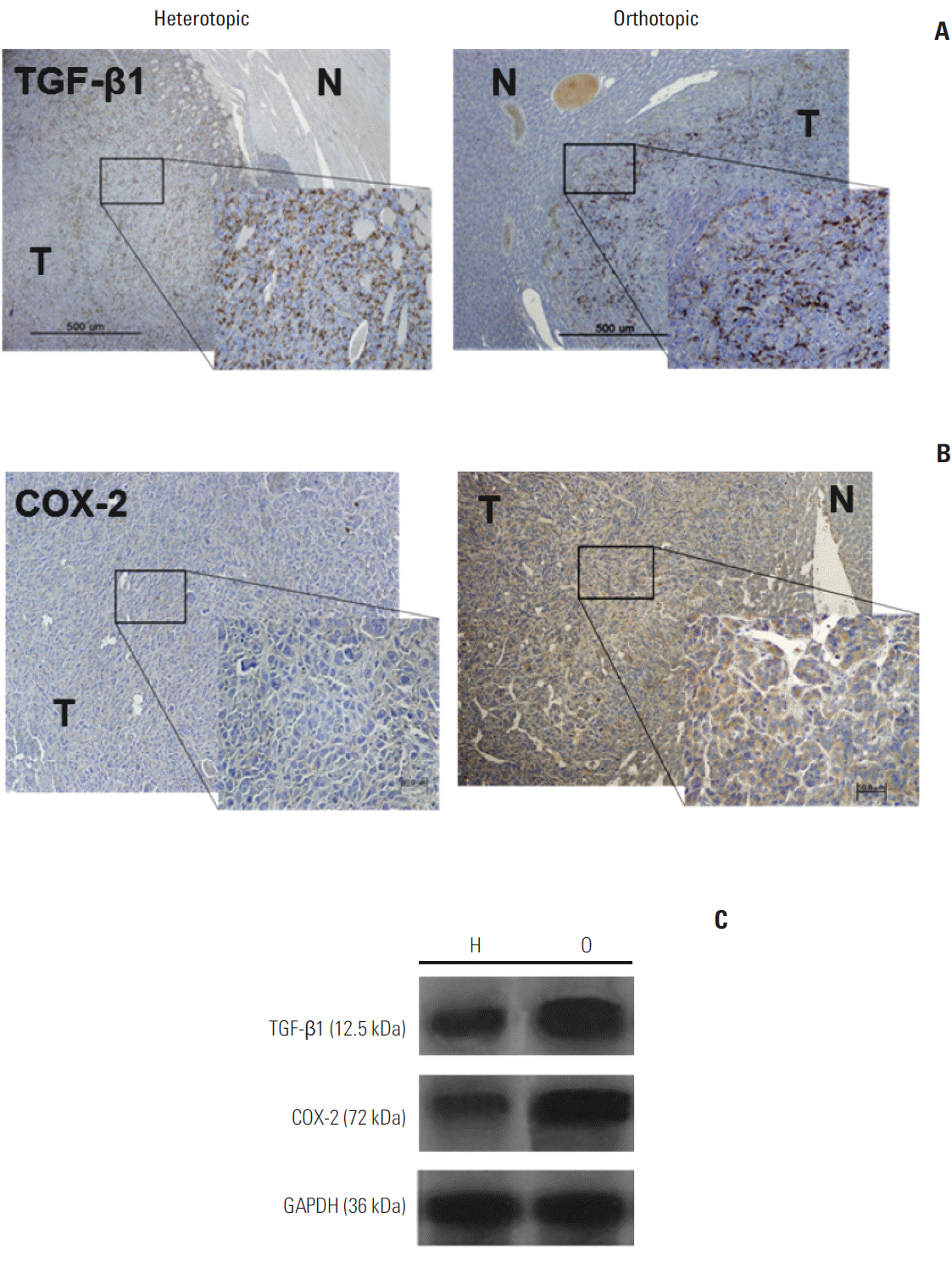 | Fig. 3.(A) Orthotopic and heterotopic tumor models demonstrated intense transforming growth factor beta1 (TGF-β1) immunoreactivity (×400). N, peritumor normal liver; T, tumor tissue. (B) The orthotopic tumor model showed predominant expression of cyclooxygenase-2 (COX-2) in the cytoplasm of peripheral tumor tissue (×400). (C) Western blot assays using antibodies against TGF-β1 and COX-2 in the orthotopic (O) and heterotopic (H) tumor models showed higher TGF-β1 and COX-2 expression in the orthotopic model than in the heterotopic tumor model. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
2. Tumor microenvironmental responses to radiation
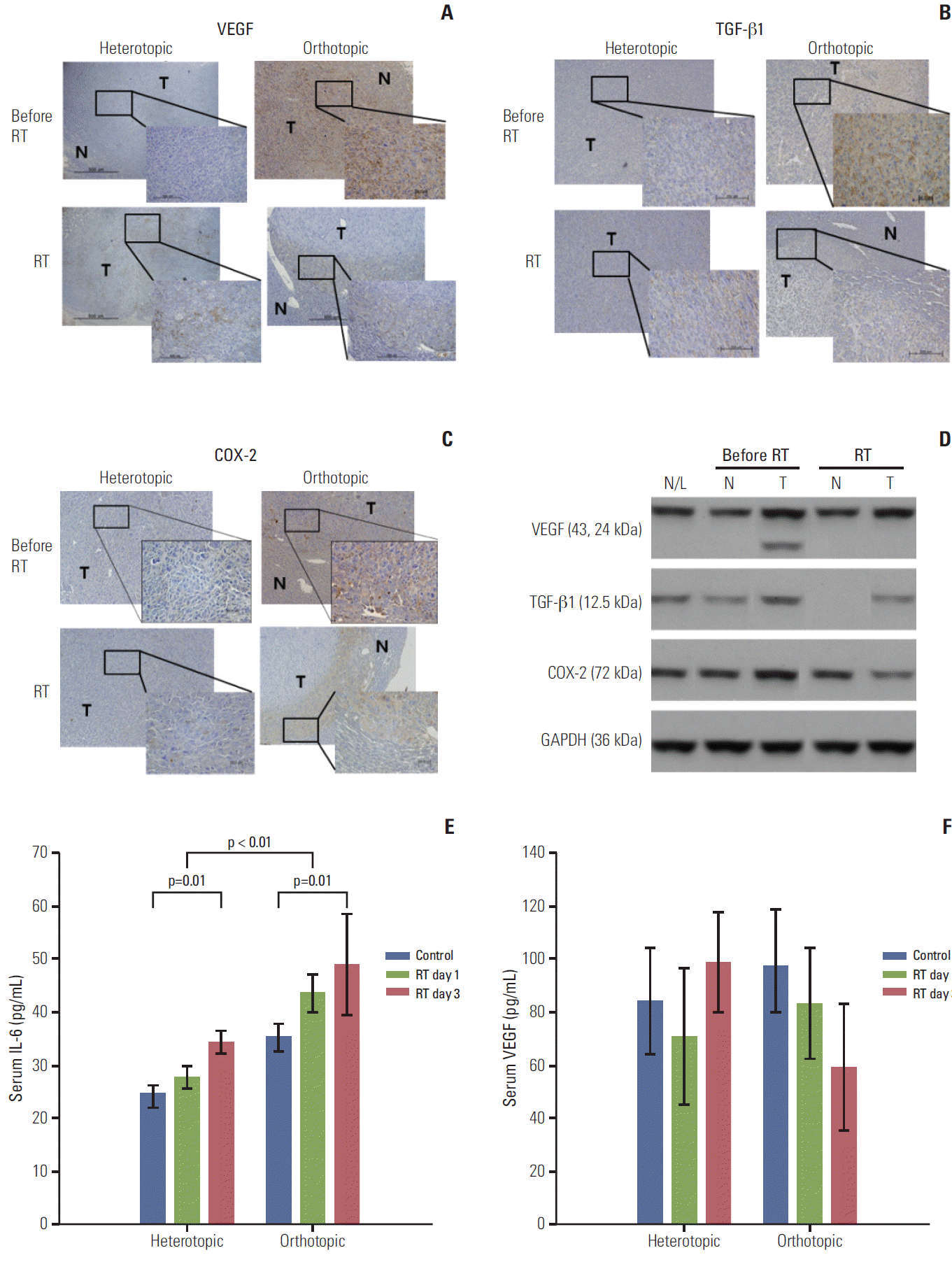 | Fig. 4.Immunohistochemical staining with antibodies against vascular endothelial growth factor (VEGF), transforming growth factor beta1 (TGF-β1), and cyclooxygenase-2 (COX-2) in the heterotopic and orthotopic tumor models (A-C). After radiation, staining for VEGF, TGF-β1, and COX-2 decreased in the orthotopic tumor model (×400), while the tumor compartment in the heterotopic tumor model showed no significant responses after radiation (3 days after radiation). (D) These findings were confirmed in the orthotopic tumor model by western blot assay. Expression of VEGF, TGF-β1, and COX-2 in tumor tissues decreased after radiation, not in peritumoral normal tissue. (E) Serum interleukin-6 (IL-6) increased after irradiation in heterotopic and orthotopic tumor models (p=0.01 and p=0.01), and the orthotopic tumor model showed a significantly higher level of IL-6 than the heterotopic tumor model (p < 0.01). Increased serum VEGF levels were also measured in the orthotopic tumor model and this level was reduced after irradiation in the orthotopic tumor model, but the difference was not significant (F). The sampling number was six per group. N/L, normal liver of naive mouse; N, normal peritumor liver; T, tumor; RT, radiation treatment; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
3. Immune cell expression after radiation
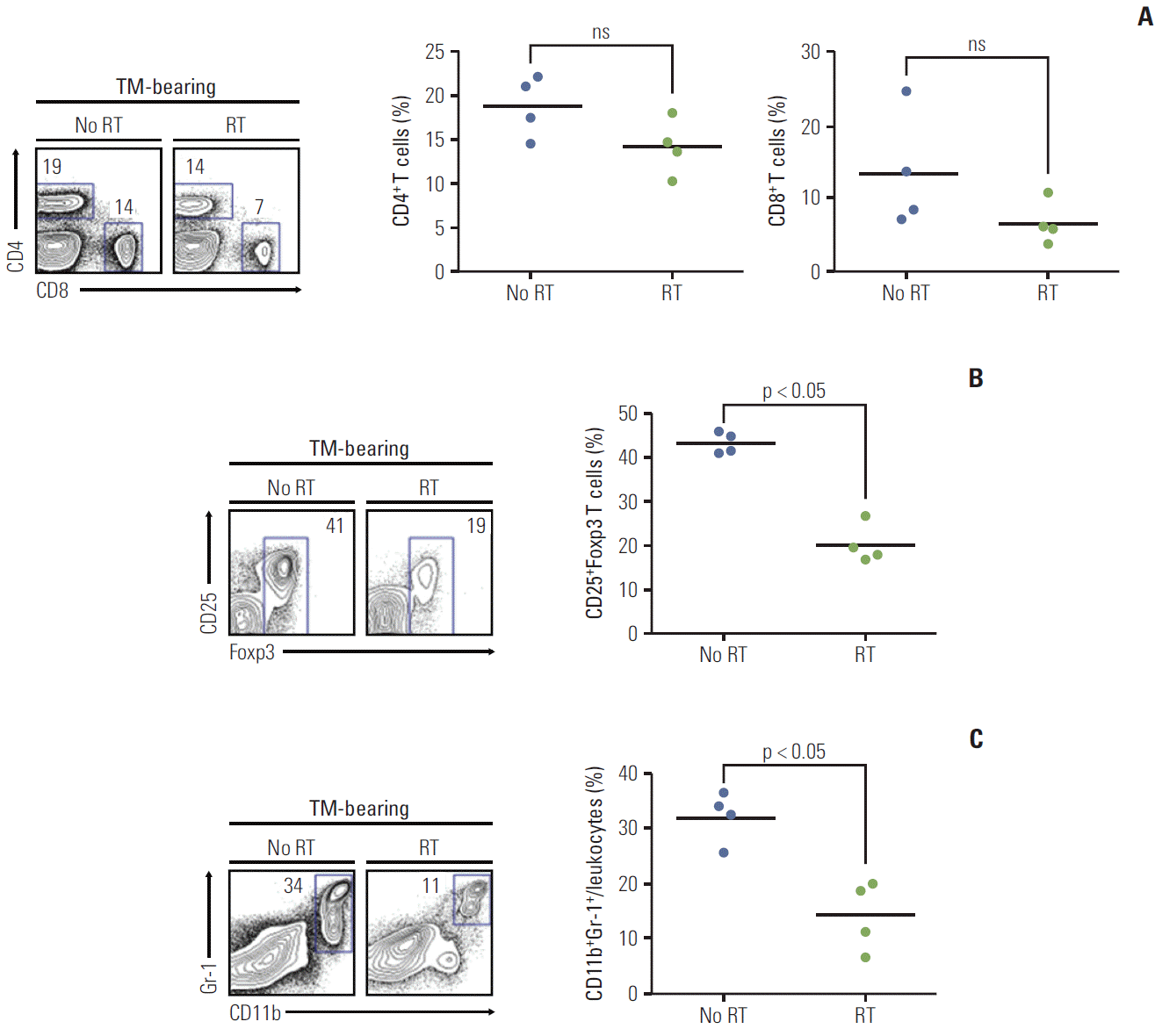 | Fig. 5.Immune cell expression after radiation (n=4 mice per each group). Irradiated orthotopic tumor samples were obtained on the third day after radiation. (A) The frequency of total CD4 and CD8 T cells in tumor-infiltrating lymphocytes are shown in the irradiated and non-irradiated orthotopic tumor model. In tumor bearing mice (TM-bearing) mice, the frequency of CD4 and CD8 T cells showed no significant difference after radiation. (B) The number of CD25+Foxp3 Treg cells in the tumor was significantly decreased after radiation (p < 0.05). (C) Myeloid-derived suppressor cell CD11b+Gr-1+ expression was also significantly decreased after radiation (p < 0.05). Treg, regulatory T cell; RT, radiation treatment; ns, not significant. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download