Abstract
Purpose
Disseminated tumor cells (DTCs) from bone marrow (BM) are a surrogate of minimal residual disease (MRD) in primary breast cancer (PBC) patients and associated with an adverse prognosis. However, BM sampling is an invasive procedure. Although there is growing evidence that circulating tumor cells (CTCs) from the blood are also suitable for monitoring MRD, data on the simultaneous detection of DTCs and CTCs are limited.
Materials and Methods
We determined the presence of DTCs using immunocytochemistry and the pan-cytokeratin antibody A45-B/B3. CTCs were determined simultaneously using a reverse transcription-polymerase chain reaction–based assay (AdnaTest Breast Cancer) and CellSearch (at least one CTC per 7.5 mL blood). We compared the detection of DTCs and CTCs and evaluated their impact on disease-free and overall survival.
Results
Of 585 patients, 131 (22%) were positive for DTCs; 19 of 202 (9%) and 18 of 383 (5%) patients were positive for CTCs, as shown by AdnaTest and CellSearch, respectively. No significant association was observed between DTCs and CTCs (p=0.248 and p=0.146 as shown by AdnaTest and CellSearch, respectively). The presence of DTCs (p=0.046) and the presence of CTCs as shown by CellSearch (p=0.007) were predictive of disease-free survival.
Conclusion
Our data confirm the prognostic relevance of DTCs and CTCs in patients with PBC. As we found no significant relationship between DTCs and CTCs, prospective trials should include their simultaneous detection. Within those trials, the question of whether or not DTCs and CTCs are independent subpopulations of malignant cell clones should be determined by molecular characterization.
Approximately 25% of patients with localized breast cancer and without axillary lymph node involvement will suffer from a systemic relapse despite successful treatment of the primary tumor. Hence, micrometastatic hematogenous spread appears to be independent from lymphatic involvement and the disease must have the ability to persist in secondary sites of the body, a phenomenon referred to as minimal residual disease (MRD).
It was hypothesized that disseminated tumor cells (DTCs), which are found in the bone marrow (BM) of 20%-40% of patients with primary breast cancer (PBC), are progenitors of subsequent metastasis and a promising marker for MRD [1]. The prognostic relevance of DTCs in PBC was demonstrated in various independent studies [2,3]. However, as DTC detection has the disadvantage of requiring an invasive procedure for sample collection, recent research has focused on the use of circulating tumor cells (CTCs) from the peripheral blood instead of BM aspiration.
Various assays, including immunocytochemistry, polymerase chain reaction (PCR)–based methods or microfluidic systems have been described for CTC detection in PBC patients with positivity rates ranging from 10% to 60% [4-8]. Currently, the most widespread approach is the CellSearch system by Veridex (Raritan, NJ), which was developed to standardize and automate immunomagnetic enrichment, immunofluorescence staining and microscopic enumeration of CTCs. Detection of CTCs using CellSearch is of prognostic significance in advanced as well as in PBC [4,5,9].
In a recent study, Schindlbeck et al. [10] compared CTC enumeration using CellSearch with DTC detection in patients with primary or metastatic breast cancer. The authors found a significant congruence between DTCs and CTCs, which increased in patients with metastases. However, in a recent study for analysis of the simultaneous detection of DTCs and CTCs in metastatic breast cancer, we were not able to confirm these results [11]. Thus, the primary goal of the current study was to compare DTCs and CTCs in patients with PBC. In addition to the enumeration of CTCs using CellSearch we used a secondary reverse transcription (RT)-PCR based assay for CTC detection.
Patients who underwent primary surgery for PBC (T1-4, N0-2, M0) at the Department of Gynecology and Obstetrics at Tuebingen University, Germany, between January 2008 and May 2014 were eligible for inclusion in this retrospective study. Exclusion criteria were recurrent or metastatic disease, bilateral breast cancer, neoadjuvant systemic therapy, R1-resection, or a previous history of secondary malignancy. While BM aspiration for DTC detection was performed using a standardized method in all patients, CTC detection was performed using either AdnaTest Breast Cancer (from January 2008 until December 2009) or CellSearch (from January 2010 until May 2014). Adjuvant therapy (including chemotherapy, endocrine therapy, trastuzumab therapy, and radiation therapy) was based on the current St. Gallen recommendations and on national treatment guidelines (http://www.ago-online.de). In addition, a significant proportion of patients received treatment with bisphosphonates. Those patients have been participating in clinical trials involving bisphosphonates (GAIN, SUCCESS A/C, NATAN, or ZOL-MRD 001), or were offered treatment with zoledronate at 4 mg every 6 months outside of clinical trials. All specimens were obtained after patients had provided written informed consent and the analysis was approved by the Ethics Committee of Tuebingen University (reference number: 560/2012R).
During primary surgery, 10-20 mL of BM aspirates were collected. Samples were processed within 24 hours. Briefly, mononuclear cells were separated via density centrifugation (1.077 g/mL; Ficoll, Biochrom, Germany), spun down onto a glass slide (Hettich cytocentrifuge, Hettich, Tuttlingen, Germany) and fixed in 4% formalin. The presence of DTCs (DTC status) was evaluated by immunostaining using the DAKO Autostainer (Dako, Glostrup, Denmark), the monoclonal mouse A45-B/B3 antibody directed against pancytokeratin (Micromet, Munich, Germany), and the DAKO-APAA detection kit (Dako). For each patient two slides (2×106 cells) were evaluated, based on consensus recommendations for standardized tumor cell detection and the criteria of the European ISHAGE Working Group [12]. An additional slide was stained using an unspecific isotype-matched antibody. In addition, with each batch of samples, leukocytes from healthy volunteers served as negative control and the cell lines MCF-7 and SKBR-3 were used as positive control.
From January 2008 until December 2009, CTCs were evaluated 1-3 days prior to surgery using AdnaTest BreastCancer (AdnaGen AG, Langenhagen, Germany). In brief, 2×5 mL of peripheral venous EDTA blood were collected using AdnaCollect tubes (AdnaGen AG). Samples were stored at 4°C and processed within 4 hours. For labeling of tumor cells, a ready-to-use mixture (AdnaTest BreastCancerSelect) containing antibodies against GA 73.3 and MUC1 was used according to the manufacturer’s instructions. The labeled cells were extracted using a magnetic particle concentrator. Subsequently, mRNA isolation from lysed, enriched cells was performed according to the manufacturer’s instructions using the Dynabeads mRNA DIRECT Micro Kit (Dynal Biotech GmbH, Hamburg, Germany) included in AdnaTest Breast-CancerDetect. For reverse transcription, Sensiscript Reverse Transcriptase (QIAGEN GmbH, Hilden, Germany) was used in combination with oligo(dT) coupled Dynabeads. Analysis of tumor-associated mRNA was performed in a multiplex PCR for human epithelial growth factor receptor 2 (HER2), MUC1, and GA 733-2. β-Actin was used as an internal PCR positive control. The primers generate fragments of the following sizes: GA 733-2, 395 base pairs (bp); MUC1, 293 bp; HER2, 270 bp; and actin, 114 bp. The PCR fragments were visualized using a 2100 Bioanalyzer (Agilent Technologies Inc., Santa Clara, CA) using DNA 1000 Lab-Chips and the Expert Software Package (ver. B.02.03.SI307). The test was considered CTC positive if a PCR fragment of at least one tumor-associated transcript (MUC-1, GA 733-2, or HER2) and a fragment of the control gene β-actin were clearly detected (peak concentration of > 0.15 ng/μL) in both blood samples.
From January 2009 until May 2014, enrichment and enumeration of CTCs was performed using the CellSearch technology (CellSearch Epithelial Cell Kit/CellSpotter Analyzer, Veridex LLC). Briefly, 7.5 mL of peripheral venous blood was collected 1-3 days prior to surgery using CellSave tubes (Veridex LLC, Warren, NJ). Samples were maintained at room temperature and proce-ssed within 72 hours. Epithelial cells were immunomagnetically enriched using antiEpCAM-coated ferrofluid. EpCAM-positive cells were then labeled with the nuclear dye 40,6-diamidino-2-phenylindole (DAPI) and monoclonal antibodies specific for the leukocyte common antigen CD45. CD45-negative, cytokeratin-positive cells with an intact nucleus were defined as CTCs and enumerated by trained operators. Blood samples containing at least one CTC per 7.5-mL blood were considered CTC-positive.
Associations between categorical variables were analyzed using chi-square and Fisher exact test. To determine survival, times from primary surgery to any recurrence of disease (disease-free survival, DFS) and death of any cause (overall survival, OS) were investigated separately. If no event occurred, data were censored at last follow-up. Patients followed up for less than 6 months were not included in the survival analysis. The influence of the DTC and CTC status, respectively, on survival was determined by univariate analysis and expressed as a hazard ratio (HR) and 95% confidence interval (CI). Kaplan-Meier curves were plotted and compared using the log-rank test. A Cox proportional regression model was used for multivariate analysis of survival. Variables were entered stepwise backward. The initial model included menopausal status, histology, tumor size, nodal status, breast cancer subtype, treatment (chemotherapy, endocrine therapy, trastuzumab therapy, and bisphosphonate therapy), CTC-status (AdnaTest and Cell-Search), and DTC-status. The effect of each variable was assessed using the Wald test and expressed as an HR and 95% CI. All statistical tests were performed using IBM SPSS Statistics ver. 20 (IBM Co., Armonk, NY) and reported two-sided with significance levels set to p < 0.05.
A total of 585 patients were included in the analysis. Patient characteristics are shown in detail in Table 1. The median age was 60 years (range, 29 to 88 years) and most women were postmenopausal (68%). The predominant histology was invasive ductal carcinoma (75%). Most patients had a tumor grade 1-2 (71%) and the tumor size was mainly less than 20 mm (pT1, 69%). The majority were nodal-negative (73%), estrogen receptor (ER)–positive (82%), progesterone receptor (PR)–positive (69%), and HER2-negative (84%).
The DTC status was determined in all 585 patients and positive in 131 of these (22%) (Table 1). DTC detection showed significant association with PR status (p=0.024) and HER2 status (p=0.040). AdnaTest Breast Cancer was used for CTC detection in 202 patients (Table 2) and 19 of these (9%) were CTC-positive. The CellSearch technology was used in 383 patients. The number of CTCs per 7.5-mL blood ranged from 0 to 42 (mean, 0.3). At least one CTC per 7.5-mL blood was detected in 18 of these patients (5%). No significant association between CTC and DTC detection was observed by the use of either AdnaTest or CellSearch (Table 3). The concordance between DTC status as compared to AdnaTest and CellSearch was 75% and 76%, respectively.
Median follow-up period (95% CI) was 35 months (range, 31 to 39 months) for all patients. For patients who underwent CTC analysis using AdnaTest and CellSearch, median follow-up period was 52 months (range, 50 to 54 months) and 22 months (range, 20 to 24 months), respectively. As shown in Fig. 1, risk of relapse was significantly greater for DTC-positive patients than DTC-negative patients (p=0.046). The impact of the DTC status on OS only reached borderlinesignificance (p=0.052). The HR (95% CI) for relapse and death was 2.13 (1.02-4.54) and 2.27 (0.97-5.32), respectively. The impact of the CTC status on survival is illustrated in Fig. 2. Use of the AdnaTest for detection of CTCs had no impact on DFS (p=0.834) or OS (p=0.270). However, in CTC analysis using CellSearch, CTC-positive patients had a significantly greater risk of relapse than CTC-negative patients (p=0.007), whereas the impact of CTC detection on OS reached borderline-significance (p=0.051). Using CellSearch, the HR (95% CI) for relapse was 6.31 (1.36-29.24) and the HR for death was 4.09 (0.88-19.01). In multivariate analysis, tumor size, nodal status, and the CTC status, as determined by CellSearch, were significant predictors of reduced DFS, whereas grading and nodal status were significant predictors of reduced OS(Table 4).
In general, prognostic and predictive biomarkers for breast cancer therapy, including ER and HER2 as well as new molecular tests like the OncoTypeDX 21-gene recurrence score, characterize tissue from the primary tumor to predict response to treatment. This approach, however, assumes that the primary tumor is representative of the entire tumor burden (including MRD) and that the initial phenotype will not change during the course of disease. There is, however, evidence that tumor cells abandon the primary lesion before acquisition of fully malignant phenotypes to undergo somatic progression at a distant site [13]. This hypothesis of early dissemination and the divergent development of the primary tumor and distant tumor cells makes DTCs and CTCs attractive candidates for monitoring and targeting the systemic component of breast cancer.
In univariate analysis, the presence of DTCs in BM significantly affected DFS. For OS only borderline significance was detected, which is most likely due to the small number of events. However, in the multivariate analysis, the DTC status had no significant impact on survival, which is in contrast to earlier studies reported by us and others [1,3]. On the one hand, this is also explained by the small sample size. On the other hand, most of the patients received bisphosphonate treatment. As described previously, bisphosphonate treatment might also contribute to the improved survival of DTC positive patients [3].
The CellSearch technology is used for detection of CTCs in translational research programs of various clinical trials. Although other, probably more sensitive methods for detection of CTCs exist [14], CellSearch provides the advantages of high reproducibility and widespread as well as automated and standardized workflow. To the best of our knowledge, this is the largest study to date comparing DTC detection in PBC with CTC detection using CellSearch. The weak correlation between CellSearch and BM sampling is in line with results from smaller trials [15,16]. However, we found that only 5% of our patients harbor at least one CTC per 7.5-mL blood, which is considerably lower than the DTC detection rate. In the large prospective multicenter SUCCESS trial, Rack et al. [5] detected at least one CTC in 22% of PBC patients; however, 30 mL blood per patient were analyzed. In addition, patients participating in the SUCCESS study were about to receive chemotherapy and thus at moderate or high risk for disease recurrence. Other studies that determined the prevalence of CTCs in PBC using CellSearch reported detection rates ranging from 9% to 24% [4,7,15,17]. These inhomogeneous results are most likely due to different patient populations, time-points of blood sampling and different sample volumes, reflecting the lack of standardization for CTC detection in PBC.
In 2004, the CellSearch system received clearance from the U.S. Food and Drug Administration as a diagnostic tool for identifying and counting CTCs of epithelial origin in patients with metastatic breast cancer. Currently, evidence that CTC detection using CellSearch is also an indicator of poor prognosis in PBC is increasing [4,5,7,17]. We confirmed that CTCs detected using CellSearch were associated with reduced DFS, although we found only borderline significance with respect to OS, which is most likely due to the short follow-up period of 22 months and the small sample size. In the SUCCESS trial, Rack et al. [5] found that the CTC status is not only predictive of prognosis at the time-point of surgery, but also after completion of adjuvant chemotherapy. Likewise, we recently demonstrated that DTC detection during or after adjuvant systemic therapy also impacts prognosis [18]. However, due to the invasiveness of BM sampling, repeated analyses of CTCs from venous blood would be more feasible for monitoring the success of adjuvant treatment.
Using AdnaTest for CTC analysis there was, again, no association with the DTC status. In contrast to CellSearch, we found no impact of the AdnaTest results on survival. In a recent study, Molloy et al. [6] compared the DTC-status of 733 PBC patients with the presence of CTCs as determined using an RT-PCR-based assay. Although the rate of CTC-positive patients was in line with our results, the authors found that CTCs were highly associated with the presence of DTCs and that both DTC- and CTC-detection were predictive of survival. However, Molloy et al. [6] used 50 mL of peripheral blood and an expression score of four marker genes (CK19, p1B, EGP, and MmG1) for detection of CTC-positive patients. Similarly, Daskalaki et al. [19] found a high concordance between BM and blood for cytokeratin-19-mRNA-positive cells in patients with PBC and both the DTC and CTC status were predictive of survival. In contrast to the study by Molloy et al. [6] and to our methodology, Daskalaki et al. [19] used RT-PCR not only for detection of CTCs but also for determination of DTCs. The antibody based detection of DTCs, however, allows for cytomorphological assessment to confirm malignancy and is therefore recommended by an international expert panel [12].
Other studies using RT-PCR based assays reported CTC-detection rates between 8% and 55% [6,19-21]. We found that the percentage of CTC-positive patients was nearly twice as high when AdnaTest was used as compared to CellSearch. A head to head comparison study between the two assays was recently conducted by Fehm et al. [22], who found a weak concordance of only 53%-64%. However, the study by Fehm et al. [22] was conducted in metastatic breast cancer patients.
Because AdnaTest and CellSearch are two completely different approaches to detection of CTCs, each method was analyzed separately. Hence, a main limitation of our study was the small sample size in each subgroup. Another limitation is the relatively short follow-up period, particularly in the subgroup analyzed using CellSearch. In addition, improved adjuvant treatment interacts with the survival analysis of this retrospectively designed analysis. We recently found that the DTC status might be predictive of the efficacy of bisphosphonate therapy [3]. A considerable proportion of patients in the current study were treated with bisphosphonates (28% of all DTC-positive women, data not shown), which might explain the small differences observed in DTC-positive versus DTC-negative patients with respect to DFS and OS. A current pilot study is evaluating the impact of denosumab on DTCs in patients with PBC (NCT01545648). In addition, HER2-targeted treatment might improve prognosis of HER2-overexpressing DTCs and CTCs, which is currently being evaluated in a phase II randomized trial for HER2-negative PBC patients with HER2-positive DTCs (NCT01779050). Hence, next to their mere quantification, characterization of DTCs and CTCs promises to identify targets for individualized breast cancer treatment. The German DETECT study group is prospectively evaluating whether characterization of CTCs in patients with metastatic breast cancer is useful in tailoring HER2-directed therapy (NCT01619111). In patients with HER2-negative PBC the ongoing TREAT-CTC study will evaluate whether CTC-positive patients will benefit from additional trastuzumab treatment (NCT0154867).
In contrast to CTC analysis in metastatic breast cancer, where CTC detection using the CellSearch technology impacts prognosis at the highest level of evidence [23], the optimal method and clinical implication for CTC analysis in PBC remains to be elucidated. The inhomogeneous prevalence of CTCs, the discordant results with respect to their correlation with the DTC status and the questionable impact on prognosis (especially when using RT-PCR based assays like AdnaTest) is most likely due to different methodologies and inhomogeneous patient characteristics. Although large prospective trials like the SUCCESS study highly support the prognostic validity of CTCs in patients with PBC, further studies are needed to standardize CTC detection and to determine their clinical potential for the personalization of breast cancer treatment.
It is not yet clear whether DTCs and CTCs represent independent compartments of MRD. CTCs have a very short estimated half-life, and therefore must be continuously replenished [24]. After successful treatment of the primary lesion, the BM might serve as a source to seed CTCs into the circulation. Although both the DTC and CTC status are predictors of an adverse prognosis, we and others found no association of CTC detection with the DTC status [15,20,25]. Thus, we do not believe that DTCs and CTCs simply represent two sides of the same coin.
To better understand their relationship, their biological role in tumor progression and their clinical role for treatment optimization, in future trials analyses of DTCs and CTCs in breast cancer patients should be performed simultaneously. Only molecular characterization of DTCs, CTCs, and tumor tissue from the same patient can ascertain whether these are independent subpopulations of malignant cell clones or in continuous cellular exchange with each other.
ACKNOWLEDGMENTS
We are grateful to Silke Duerr-Störzer, Ingrid Teufel, Sabine Hofmeister, Angelika Amman, and Brigitte Neth for providing excellent technical assistance.
References
1. Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005; 353:793–802.

2. Gebauer G, Fehm T, Merkle E, Beck EP, Lang N, Jager W. Epithelial cells in bone marrow of breast cancer patients at time of primary surgery: clinical outcome during long-term follow-up. J Clin Oncol. 2001; 19:3669–74.

3. Hartkopf AD, Taran FA, Wallwiener M, Hahn M, Becker S, Solomayer EF, et al. Prognostic relevance of disseminated tumour cells from the bone marrow of early stage breast cancer patients: results from a large single-centre analysis. Eur J Cancer. 2014; 50:2550–9.
4. Lucci A, Hall CS, Lodhi AK, Bhattacharyya A, Anderson AE, Xiao L, et al. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 2012; 13:688–95.

5. Rack B, Schindlbeck C, Juckstock J, Andergassen U, Hepp P, Zwingers T, et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst. 2014; 106:dju066.

6. Molloy TJ, Bosma AJ, Baumbusch LO, Synnestvedt M, Borgen E, Russnes HG, et al. The prognostic significance of tumour cell detection in the peripheral blood versus the bone marrow in 733 early-stage breast cancer patients. Breast Cancer Res. 2011; 13:R61.

7. Franken B, de Groot MR, Mastboom WJ, Vermes I, van der Palen J, Tibbe AG, et al. Circulating tumor cells, disease recurrence and survival in newly diagnosed breast cancer. Breast Cancer Res. 2012; 14:R133.

8. Nora Dickson M, Tsinberg P, Tang Z, Bischoff FZ, Wilson T, Leonard EF. Efficient capture of circulating tumor cells with a novel immunocytochemical microfluidic device. Biomicrofluidics. 2011; 5:34119.

9. Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004; 351:781–91.

10. Schindlbeck C, Andergassen U, Hofmann S, Juckstock J, Jeschke U, Sommer H, et al. Comparison of circulating tumor cells (CTC) in peripheral blood and disseminated tumor cells in the bone marrow (DTC-BM) of breast cancer patients. J Cancer Res Clin Oncol. 2013; 139:1055–62.

11. Hartkopf AD, Stefanescu D, Wallwiener M, Hahn M, Becker S, Solomayer EF, et al. Tumor cell dissemination to the bone marrow and blood is associated with poor outcome in patients with metastatic breast cancer. Breast Cancer Res Treat. 2014; 147:345–51.

12. Fehm T, Braun S, Muller V, Janni W, Gebauer G, Marth C, et al. A concept for the standardized detection of disseminated tumor cells in bone marrow from patients with primary breast cancer and its clinical implementation. Cancer. 2006; 107:885–92.

13. Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009; 9:302–12.

14. Fischer JC, Niederacher D, Topp SA, Honisch E, Schumacher S, Schmitz N, et al. Diagnostic leukapheresis enables reliable detection of circulating tumor cells of nonmetastatic cancer patients. Proc Natl Acad Sci U S A. 2013; 110:16580–5.

15. Krishnamurthy S, Cristofanilli M, Singh B, Reuben J, Gao H, Cohen EN, et al. Detection of minimal residual disease in blood and bone marrow in early stage breast cancer. Cancer. 2010; 116:3330–7.

16. Slade MJ, Payne R, Riethdorf S, Ward B, Zaidi SA, Stebbing J, et al. Comparison of bone marrow, disseminated tumour cells and blood-circulating tumour cells in breast cancer patients after primary treatment. Br J Cancer. 2009; 100:160–6.

17. Bidard FC, Mathiot C, Delaloge S, Brain E, Giachetti S, de Cremoux P, et al. Single circulating tumor cell detection and overall survival in nonmetastatic breast cancer. Ann Oncol. 2010; 21:729–33.

18. Gruber I, Fehm T, Taran FA, Wallwiener M, Hahn M, Wallwiener D, et al. Disseminated tumor cells as a monitoring tool for adjuvant therapy in patients with primary breast cancer. Breast Cancer Res Treat. 2014; 144:353–60.

19. Daskalaki A, Agelaki S, Perraki M, Apostolaki S, Xenidis N, Stathopoulos E, et al. Detection of cytokeratin-19 mRNA-positive cells in the peripheral blood and bone marrow of patients with operable breast cancer. Br J Cancer. 2009; 101:589–97.

20. Benoy IH, Elst H, Philips M, Wuyts H, Van Dam P, Scharpe S, et al. Real-time RT-PCR detection of disseminated tumour cells in bone marrow has superior prognostic significance in comparison with circulating tumour cells in patients with breast cancer. Br J Cancer. 2006; 94:672–80.

21. Stathopoulou A, Vlachonikolis I, Mavroudis D, Perraki M, Kouroussis C, Apostolaki S, et al. Molecular detection of cytokeratin-19-positive cells in the peripheral blood of patients with operable breast cancer: evaluation of their prognostic significance. J Clin Oncol. 2002; 20:3404–12.

22. Fehm T, Muller V, Aktas B, Janni W, Schneeweiss A, Stickeler E, et al. HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res Treat. 2010; 124:403–12.

23. Bidard FC, Peeters DJ, Fehm T, Nole F, Gisbert-Criado R, Mavroudis D, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014; 15:406–14.

Fig. 1.
Impact of disseminated tumor cells (DTCs) from bone marrow on disease-free survival (A) and overall survival (B).
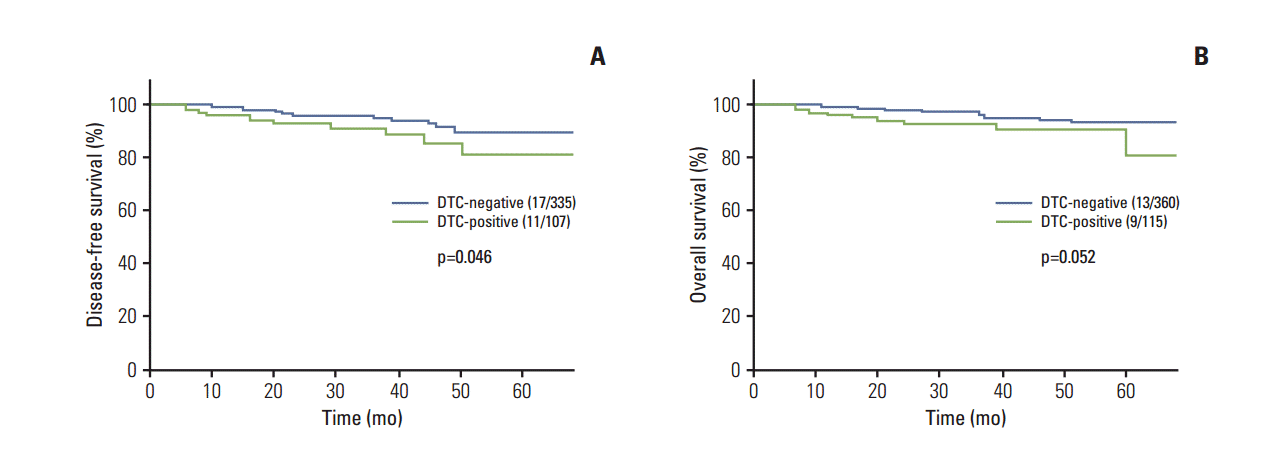
Fig. 2.
Impact of circulating tumor cells (CTCs) from peripheral blood as determined by AdnaTest (A, B) and CellSearch (C, D) on disease-free survival (A, C) and overall survival (B, D).
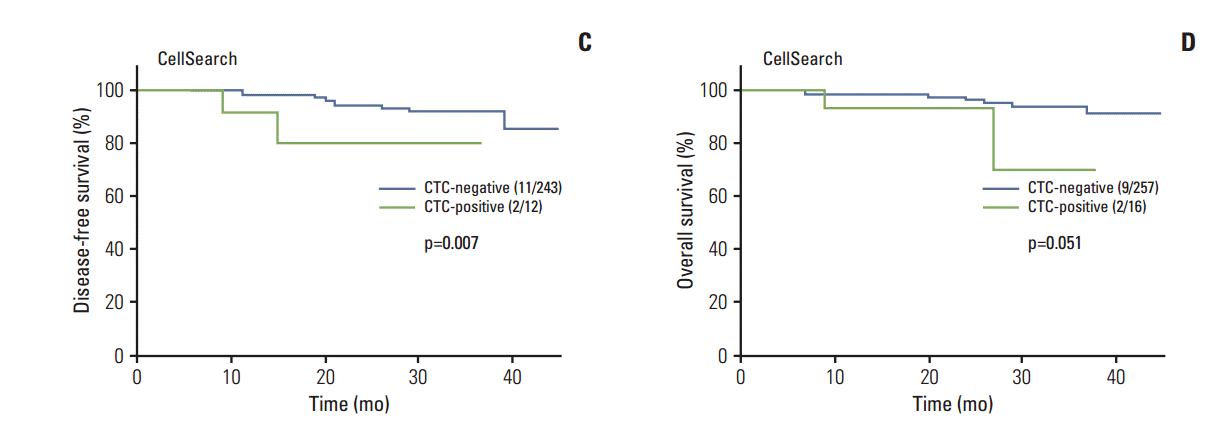
Table 1.
Disseminated tumor cell status and patient characteristics
Table 2.
Circulating tumor cell status and patient characteristics
Table 3.
Comparison of disseminated and circulating tumor cell status
Table 4.
Multivariate analysis of disease-free survival and overall survival
| Parameter |
DFS |
OS |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Grading | ||||||
| G1-2 | - | - | NS | 1.00 | 1.66-14.66 | 0.004 |
| G3 | - | 4.94 | ||||
| Tumor size | ||||||
| T1 | 1.00 | 1.66-9.36 | 0.002 | - | - | NS |
| T2-4 | 3.94 | - | ||||
| Nodal status | ||||||
| Negative | 1.00 | 1.41-8.25 | 0.007 | 1.00 | 1.36-9.55 | 0.010 |
| Positive | 3.41 | 3.60 | ||||
| CTC status | ||||||
| Negative | 1.00 | 1.43-38.58 | 0.018 | - | - | NS |
| Positivea) | 7.39 | - | ||||




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download