Abstract
Purpose
The purpose of this study was to investigate the clinicopathological features of pulmonary metastasis from cervical cancer.
Materials and Methods
We reviewed the medical records of 56 patients with cervical cancer who developed pulmonary metastasis after radical hysterectomy, postoperative concurrent chemoradiation or systemic chemotherapy between January 1990 and March 2014.
Results
Fifty-six patients were diagnosed with pulmonary metastasis from cervical cancer. The prevalence of pulmonary metastasis was 3.6%. The mean event-free duration was 12 months. Twelve patients underwent surgical removal of metastatic lesions. The overall survival (OS) of patients with ≤ 3 metastatic lung lesions was 40.7 months, longer than those with > 4 lesions (25 months, p=0.034). The OS of patients who underwent surgical resection was 53.8 months, longer than that of those who did not (p=0.006). In addition, the OS of patients with adjuvant platinum-based chemotherapy was 32.6 months (p=0.027).
The mortality of cervical cancer has decreased since cervical cancer screening tests, such as Papanicolaou smear, were introduced, but it remains a significant cause of death [1,2]. About 7,000-15,000 new patients are diagnosed with cervical cancer every year and 4,600-6,800 patients die of cervical cancer every year worldwide [2-4]. About 10%-20% of patients develop recurrent disease after primary treatment. The treatment of recurrent disease may differ by the site of recurrence, prior treatment, and degree of recurrence [1]. The lung is commonly affected by hematogenous spread, often detected as a solitary pulmonary nodule or multiple metastases. Especially in cases of a solitary pulmonary nodule, it is important to discriminate between pulmonary metastasis, primary lung cancer, and carcinoid tumors [5].
The literature has led many investigators to focus on surgery upon a solitary pulmonary nodule and its outcome. However, multiple pulmonary metastases are frequent. In this study, we reviewed the medical records of cervical cancer patients who were diagnosed with lung metastases (solitary or multiple) during or after primary treatment and analyzed their clinicopathological characteristics.
We analyzed 56 patients with cervical cancer who were treated with radical hysterectomy, concurrent chemoradiation, or combination chemotherapy, and developed pulmonary metastases during or after primary treatment between January 1990 and March 2014. Clinicopathological data was collected from their medical records. The records include demographic characteristics, the initial stage, symptoms at the diagnosis of pulmonary metastasis, event-free duration (EFD), and survival time. This study was approved by the Institutional Review Board (KC14RISI0487). All statistical analyses were done using the Wilcoxon sign rank, chi-square, log-rank, and ANOVA tests. SPSS ver. 22.0 (IBM Co., Armonk, NY) was used for all statistical analyses.
The median age was 55 years, with a range of 36 to 61 years. Ten patients (17.9%) were at stage I (4 patients were at stage Ib1 and 6 patients were at stage Ib2), 29 patients (51.8%) at stage II, six patients (10.1%) at stage III, 10 patients (17.9%) at stage IV, and one patient’s (1.8%) stage was unknown. Patients at stage Ib1 were observed further without any additional treatment. Of the six patients at stage Ib2, four underwent concurrent chemoradiation therapy (CCRT) for lymph node metastasis and two with suspicious lymphatic invasion who refused treatment were tracked for outcomes. The remaining patients underwent operation, CCRT, or chemotherapy depending on tumor stage. Forty-two patients out of the 56 underwent primary or postoperative CCRT. CCRT was cisplatin-based (40 mg/m2 at 7-day intervals). Forty-six patients, excluding patients at stage I, underwent combination chemotherapy every 3 weeks as a primary treatment or after CCRT depending on tumor stage: carboplatin (300 mg/m2)+VP16 (100 mg/m2), carboplatin (area under the curve 5-6 mg/m2)+paclitaxel (175 mg/m2), or cisplatin (50 mg/m2)+5-fluorouracil (1,000 mg/m2). When lung metastasis was suspected during exam at the outpatient clinic, immunohistochemistry staining of the resected or biopsied specimens for cytokeratin 7, CD56a, chromogranin, thyroid transcription factor-1, and p16 were conducted to rule out primary lung cancer. Based on the immunohistochemical staining results, specimens that were diffusely stained and strongly positive for p16 diffuse and strong positive were included in the study. We excluded specimens that were focally stained and weakly positive for p16. Table 1 shows the characteristics of the study subjects. Although a significant portion of the patients (40 patients, 71.4%) did not have pulmonary symptoms at recurrence, their pulmonary recurrence or metastasis was incidentally detected by chest X-ray or pelvic computed tomography (CT). Four patients (7.1%) complained of dyspnea, four patients (7.1%) of general ache or weakness, and eight patients (14.4%) of dry cough. The histological findings of primary cervical cancer showed 45 patients with squamous cell carcinoma (SCC), seven patients with adenocarcinoma, three patients with adenosquamous cell carcinoma, and one patient with small cell carcinoma. The pulmonary lesions were noticed in the right lung (n=10, 17.9%), the left lung (n=5, 8.9%), and both lungs (n=37, 66.1%). However, four patients (7.1%) had malignant pleural effusion without parenchymal involvement. Twelve patients underwent complete removal of lung metastasis: six had one metastatic nodule, four had two metastatic nodules, and two had three metastatic nodules. Of the remaining 44 patients, four with malignant pleural effusion underwent paracentesis to relieve dyspnea, and 40 underwent biopsy (bronchoscopic biopsy, CT guided biopsy, open lung biopsy) because the pulmonary metastatic lesions were small and multiple. The mean EFD (duration from the diagnosis of initial cervical cancer to lung metastasis) was 12 months. Table 2 shows EFD and overall survival (OS) by various factors. EFD did not differ significantly by initial stage, but OS decreased by initial stage (p < 0.001). The survival curve using the log-rank test was similar (p=0.001) (Fig. 1). OS and EFD did not differ significantly by histologic type (p=0.141 and p=0.234, respectively) (Table 2, Fig. 2).
Our 52 patients received platinum-based chemotherapy after pulmonary lesions were resected or pulmonary metastases were diagnosed by radiologic imaging studies. Of patients diagnosed with pulmonary metastases, four refused additional treatment. OS and EFD were longer in patients treated with platinum-based chemotherapy.
The number of pulmonary lesions at diagnosis on chest CT was significantly associated with OS and EFD. Twenty-two patients, including three patients with malignant pleural effusion, had 0 to 3 nodules in the chest by CT. Thirty-four patients showed multiple variable-sized nodules. OS and EFD were longer in patients with ≤ 3 nodules than in those with > 4 nodules (p=0.034 and p=0.045 , respectively). Of 22 patients who had ≤ 3 nodules, 12 were treated with surgical resection (10 patients, mass excision; 2 patients, lobectomy). Three patients were treated with drainage of pleural effusion and pleurodesis. No surgical treatment was conducted on seven patients: two with the lesions in the hilar area, in one with a poor general condition and low operability, and in four with extrapulmonary metastatic lesions. OS was longer in patients who were treated with surgical resection than in those who were not (p=0.006).
Pulmonary metastasis occurs in a small number of patients with cervical cancer. The prevalence of pulmonary metastasis was 2.1%-6.1% [2,6-8]. The relationship between the incidence of pulmonary metastasis and the initial stage is extremely weak. In our study, 17.9% of the patients were at stage I, 51.8% at stage II, 10.1% at stage III, 17.9% at stage IV, and 1.8% at an unknown stage. Recurrence occurred most frequently in patients at stage II and decreased in those at stages III and IV. Barter et al. [2] also reported that pulmonary metastasis is not associated with the initial stage. By their report, 36% of the patients were at stage I, 33% at stage II, 11% at stage III, and 15% at stage IV. In contrast, Imachi et al. [6] demonstrated that the incidence of pulmonary metastasis is high when the initial stage is advanced and that the size of primary cancer is related to the incidence of pulmonary metastasis.
In our study most patients did not complain of pulmonary symptoms. Pulmonary lesions were detected on routine follow-up chest X-ray or pelvic CT. The mean EFD was 12 months. In previous studies, the mean EFD was 24 months [9,10]. Since many patients with pulmonary metastasis have no specific symptoms, they should receive regular and long-term chest exams [11]. Serological tumor markers, such as SCC and carcinoembryonic antigen (CEA), can detect tumor recurrence. Rose et al. [12] and Ngan et al. [13] indicated that SCC levels are higher in the squamous cell tumors than in the non-squamous cell tumors and decreased after treatment. In our study, we investigated the association between tumor markers at the diagnosis of metastasis and survival, using the Spearman correlation coefficient, suggesting that as SCC or CEA levels are increased, OS becomes shorter (p=0.008 and p=0.006, respectively).
In this study, metastatic lesions were treated with surgical resection, chemotherapy or radiation. The decision to perform surgery should be based on the location and number of metastatic lesions, and general condition. Many investigators treat metastatic lesions with resection and adjuvant chemotherapy, especially for a solitary pulmonary nodule [1,7,14-16]. Their criteria for surgical treatment include the following: (1) complete removal or control of primary lesions, (2) no evidence of extrapulmonary lesions, (3) patient tolerance of surgery, (4) sufficient pulmonary reserve after resection, and (5) no treatment better than surgery [1,14-17]. In our study, 21.4% of all patients underwent operation (17.9% mass excision, 3.5% lobectomy). The mean number of the resected lesions was 1.8, the mean size was 3.3 cm, and negative resection margins were observed in all patients.
When pulmonary nodules are found on chest X-ray or CT, it is important to discriminate between primary and metastatic lesions. Discrimination between primary pulmonary SCC and metastatic pulmonary SCC from the cervix is important in therapeutic strategy for SCC [4]. To differentiate between primary pulmonary SCC and lung metastatic pulmonary SCC from the cervix, p16 immunohistochemical staining can be used. The marker p16 is a member of the INK4a family and acts as a cyclin dependent inhibitor. It maintains the tumor suppressor activity of pRb. Human papillomavirus (HPV) infection changes cell cycle regulation through degradation of pRb and causes the development of carcinoma. It rarely occurs in primary pulmonary SCC [4]. Wang et al. [4] reported that immunohistochemical staining was positive for p16 in 98% of patients with metastatic pulmonary SCC from cervix, which was diffusely and strongly stained. However, in 21% of patients with primary pulmonary SCC, immunohistochemical staining was also positive for p16, which was focally or weakly stained. Plaza et al. [3] distinguished these disease entities using reverse transcription in situ polymerase chain reaction to detect HPV. In our study, we also used immunohistochemical staining for p16. Pulmonary metastasis is related to prognostic factors, including EFD, the number of lung masses, radiographic patterns of pulmonary metastasis, and cell types (Table 3). Yamamoto et al. [1] and Barter et al. [2] indicate that EFD does not influence survival. In contrast, Fuller et al. [16] report that longer EFD (> 36 months) is associated with good prognosis. Anderson et al. [7] observe that long EFD (> 12 months) is associated with good prognosis. In our study, OS was more prolonged in patients with longer EFD. In particular, OS abruptly increased after EFD reached 24 months (Fig. 3). The number of resected lung masses does not influence prognosis [14,18]. Girard et al. [19] report that the number of resection mass does not influence prognosis, whereas resectability of pulmonary masses is associated with a good prognosis. In our study, OS was significantly longer in patients treated with surgical resection than in those who were not (Fig. 4). Radiographic patterns of pulmonary metastasis influence prognosis. Barter et al. [2] suggest that the right lung is involved more frequently than the left lung and that mediastinal and hilar involvements lead to poorer prognosis than parenchymal involvement. Cell types influence prognosis. Yamamoto et al. [1] report that SCC shows a better prognosis than non-SCC, such as adenocarcinoma and adenosquamous cell carcinoma. Imachi et al. [6] also report that the incidence of pulmonary metastasis and positivity in peritoneal cytology are higher in the adenocarcinoma group than in the SCC group. In our study, however, OS and EFD did not differ significantly by the histologic type. The reason for this may be that the number of non-squamous cell tumor cases was smaller than that of squamous cell tumor cases.
Many clinicians are currently treating patients with pulmonary metastasis using platinum. Response rates to platinum-based chemotherapy are reported to be 26%-67.7% [2,20]. The response rates to cytoxan and adriamycin are reported to be 65% and 16%-40%, respectively [2,8,21]. In our study, patients were treated with platinum-based combination chemotherapy, with a response rate of 69.6%.
Although hematogenous spread of primary tumors more often occurs in the lung rather than the brain and liver, pulmonary metastases from cervical cancer is rare. Patients with pulmonary metastases often present nonspecific symptoms and sometimes no symptoms. We reviewed the medical records of patients with cervical cancer with pulmonary metastases during or after treatment and analyzed clinical and histopathological characteristics. This study is limited by its retrospective design. Further studies with a variable design are warranted.
References
1. Yamamoto K, Yoshikawa H, Shiromizu K, Saito T, Kuzuya K, Tsunematsu R, et al. Pulmonary metastasectomy for uterine cervical cancer: a multivariate analysis. Ann Thorac Surg. 2004; 77:1179–82.

2. Barter JF, Soong SJ, Hatch KD, Orr JW, Shingleton HM. Diagnosis and treatment of pulmonary metastases from cervical carcinoma. Gynecol Oncol. 1990; 38:347–51.

3. Plaza JA, Ramirez NC, Nuovo GJ. Utility of HPV analysis for evaluation of possible metastatic disease in women with cervical cancer. Int J Gynecol Pathol. 2004; 23:7–12.

4. Wang CW, Wu TI, Yu CT, Wu YC, Teng YH, Chin SY, et al. Usefulness of p16 for differentiating primary pulmonary squamous cell carcinoma from cervical squamous cell carcinoma metastatic to the lung. Am J Clin Pathol. 2009; 131:715–22.

5. Ge J, Gou HF, Chen Y, Cheng K, Li LH, Dong H, et al. Clinical characteristics of patients with solitary pulmonary mass after radical treatment for primary cancers: pulmonary metastasis or second primary lung cancer? Cancer Invest. 2013; 31:397–403.

6. Imachi M, Tsukamoto N, Matsuyama T, Nakano H. Pulmonary metastasis from carcinoma of the uterine cervix. Gynecol Oncol. 1989; 33:189–92.

7. Anderson TM, McMahon JJ, Nwogu CE, Pombo MW, Urschel JD, Driscoll DL, et al. Pulmonary resection in metastatic uterine and cervical malignancies. Gynecol Oncol. 2001; 83:472–6.

8. Carlson V, Delclos L, Fletcher GH. Distant metastases in squamous-cell carcinoma of the uterine cervix. Radiology. 1967; 88:961–6.

9. Ito H, Shigematsu N, Kawada T, Kubo A, Isobe K, Hara R, et al. Radiotherapy for centrally recurrent cervical cancer of the vaginal stump following hysterectomy. Gynecol Oncol. 1997; 67:154–61.

10. Krebs HB, Helmkamp BF, Sevin BU, Poliakoff SR, Nadji M, Averette HE. Recurrent cancer of the cervix following radical hysterectomy and pelvic node dissection. Obstet Gynecol. 1982; 59:422–7.

11. Shiromizu K, Kasamatsu T, Takahashi M, Kikuchi A, Yoshinari T, Matsuzawa M. A clinicopathological study of postoperative pulmonary metastasis of uterine cervical carcinomas. J Obstet Gynaecol Res. 1999; 25:245–9.

12. Rose PG, Baker S, Fournier L, Nelson BE, Hunter RE. Serum squamous cell carcinoma antigen levels in invasive cervical cancer: prediction of response and recurrence. Am J Obstet Gynecol. 1993; 168:942–6.

13. Ngan HY, Cheng GT, Cheng D, Wong LC, Ma HK. Posttreatment serial serum squamous cell carcinoma antigen (SCC) in the monitoring of squamous cell carcinoma of the cervix. Int J Gynecol Cancer. 1996; 6:115–9.

14. Anraku M, Yokoi K, Nakagawa K, Fujisawa T, Nakajima J, Akiyama H, et al. Pulmonary metastases from uterine malignancies: results of surgical resection in 133 patients. J Thorac Cardiovasc Surg. 2004; 127:1107–12.

15. Pastorino U, Buyse M, Friedel G, Ginsberg RJ, Girard P, Goldstraw P, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997; 113:37–49.

16. Fuller AF Jr, Scannell JG, Wilkins EW Jr. Pulmonary resection for metastases from gynecologic cancers: Massachusetts General Hospital experience, 1943-1982. Gynecol Oncol. 1985; 22:174–80.

18. Seki M, Nakagawa K, Tsuchiya S, Matsubara T, Kinoshita I, Weng SY, et al. Surgical treatment of pulmonary metastases from uterine cervical cancer: operation method by lung tumor size. J Thorac Cardiovasc Surg. 1992; 104:876–81.
19. Girard P, Baldeyrou P, Le Chevalier T, Lemoine G, Tremblay C, Spielmann M, et al. Surgical resection of pulmonary metastases. Up to what number? Am J Respir Crit Care Med. 1994; 149:469–76.

20. Alberts DS, Martimbeau PW, Surwit EA, Oishi N. MitomycinC, bleomycin, vincristine, and cis-platinum in the treatment of advanced, recurrent squamous cell carcinoma of the cervix. Cancer Clin Trials. 1981; 4:313–6.

21. Wallace HJ Jr, Hreshchyshyn MM, Wilbanks GD, Boronow RC, Fowler WC Jr, Blessing JA. Comparison of the therapeutic effects of adriamycin alone versus adriamycin plus vincristine versus adriamycin plus cyclophosphamide in the treatment of advanced carcinoma of the cervix. Cancer Treat Rep. 1978; 62:1435–41.
Fig. 1.
Overall survival decreases by the initial stage in pulmonary metastasis (p=0.001, the log-rank test).
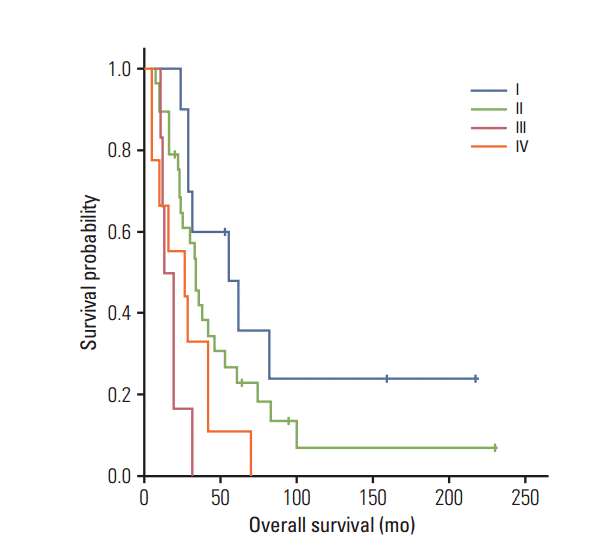
Fig. 2.
Overall survival by the histologic type. There is no significant difference between squamous cell and non-squamous cell type (log-rank test, p=0.2).
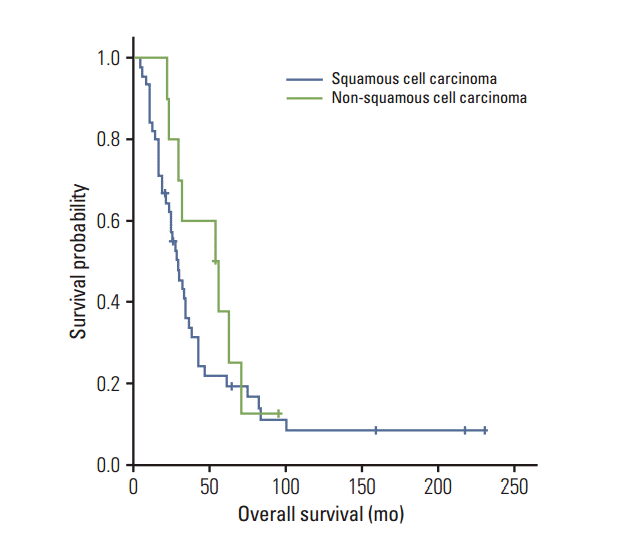
Fig. 3.
The relationship between event-free duration (EFD) and overall survival. Overall survival becomes longer with increasing EFD. Overall survival increases abruptly after EFD reaches 24 months (p=0.019).
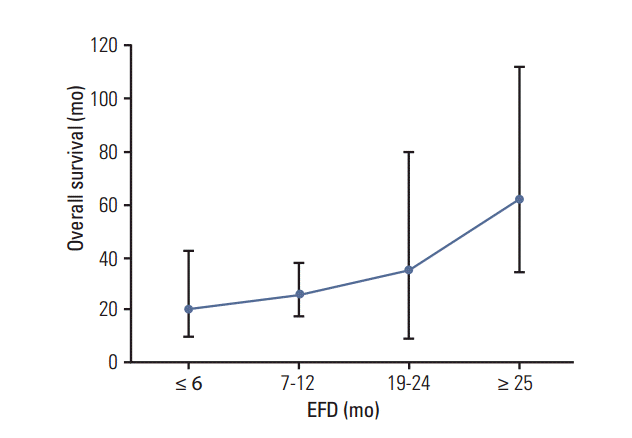
Fig. 4.
Overall survival by resectability. The resection
group has better overall survival than the non-resection group (log-rank test, p=0.025).
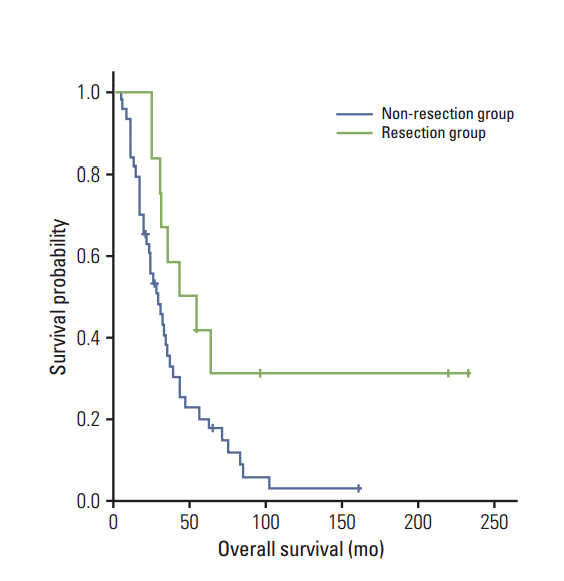
Table 1.
Patient characteristics
Table 2.
Overall survival and event-free duration
| Factor | No. | Overall survival (95% CI, mo) | p-value | Event-free duration (95% CI, mo) | p-value |
|---|---|---|---|---|---|
| Stage | |||||
| I | 10 | 56.3 (33-96) | < 0.001 | 18.1 (10.2-32) | 0.297 |
| II | 29 | 32.9 (24.6-43.9) | 12.6 (9.7-16.3) | ||
| III | 6 | 16.2 (10.4-25.2) | 12.2 (9-16.6) | ||
| IV | 10 | 18.8 (8.8-40.4) | 20.7 (9.5-44.7) | ||
| Histology | |||||
| Squamous cell | 45 | 28 (21.4-36.5) | 0.141 | 13.3 (10.7-16.5) | 0.234 |
| Adenocarcinoma | 7 | 57.8 (39.2-85.3) | 21.9 (12.1-39.7) | ||
| Other | 4 | 29.7 (15.6-56.5) | 13.8 (6.3-30.5) | ||
| Adjuvant chemotherapy after diagnosis | |||||
| Yes | 52 | 32.6 (25.8-41.2) | 0.027 | 15.2 (12.8-18.2) | 0.006 |
| No | 4 | 12.5 (6.6-23.7) | 6 (1.4-26.5) | ||
| Detection number of metastatic pulmonary lesion | |||||
| 0-3a) | 22 | 40.7 (27.7-60.0) | 0.035 | 17.6 (12.9-24.2) | 0.045 |
| ≥ 4 | 34 | 25 (19-33) | 12.1 (9.6-15.2) | ||
| Surgical resection | |||||
| Yes | 12 | 53.8 (32.8-88.4) | 0.006 | 17.2 (12.9-22.9) | 0.255 |
| No | 44 | 25.9 (20.3-33.2) | 13.3 (10.5-16.8) |
Table 3.
Survival and important prognostic factors from the literature
| Source | No. of patients | Initial stage | Survival | Important prognostic factors |
|---|---|---|---|---|
| Yamamoto et al. [1] | 7,748 | Ib-II | 32.9% a) | Histologic type |
| Age | ||||
| Number of metastasis | ||||
| Barter et al. [2] | 88 | I-IV | 2.3%a) | Platinum-based chemotherapy |
| Anraku et al. [14] | 71 (cervical cancer) | 54.6%a) | Resectability of lung lesions | |
| 62 (uterine body cancer) | Disease free interval | |||
| Fuller et al. [16] | 15 | I-IV | 36%a) | Event-free duration |
| Resectability of lung lesions | ||||
| Shiromizu et al. [11] | 519 | Ib-IIb | 36%a) | Platinum-based chemotherapy |
| Tumor marker | ||||
| Resectability of lung lesions | ||||
| Present study | 56 | I-IV | 32.8 mob) | Adjuvant chemotherapy |
| Detection number of pulmonary metastatic lesion | ||||
| Resectability of lung lesions |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download