Abstract
Purpose
The purpose of this study was to calculate the operating characteristics of narrowband imaging (NBI) cystoscopy versus traditional white light cystoscopy (WLC) in common clinical scenarios involving suspicion of bladder urothelial carcinoma (UC).
Materials and Methods
Sixty-three consecutive patients initially underwent WLC and then NBI in a single session for evaluation of microscopic hematuria (group I, n=20), gross hematuria (group II, n=19), and follow-up for prior UC (group III, n=24), by an experienced urologist. All lesions that were abnormal in contrast with adjacent normal mucosa were diagnosed as positive and biopsied.
Results
Sixty-six biopsies from 47 patients were performed. Pathologic examination showed 17 cases of UC from 21 sites. While the overall sensitivity of NBI was similar to that of WLC (100% vs. 94.1%), the specificity of NBI was significantly lower than that of WLC (50% vs. 86.9%, p < 0.001), particularly in group III (38.9% vs. 88.9%, p=0.004). Based on identification by NBI only, 23 additional biopsies from 18 cases were performed for identification of one patient with UC, who belonged to group III. In this group, to identify this specific patient, 15 additional biopsies were performed from 10 patients. All seven cases with positive findings from NBI within 2 months after the last intravesical therapy were histologically proven as negative.
The role of cystoscopic imaging is critical in detection and surveillance of nonmuscle invasive bladder cancer (NMIBC), including Ta/T1 urothelial carcinoma and carcinoma in situ (CIS). However, accuracy in diagnosis using standard white light cystoscopy (WLC) is unsatisfactory, leading to a high recurrence rate, mainly due to overlooked tumors [1], despite routine administration of postoperative intravesical therapy [2]. To overcome this drawback, the novel technology of narrow band imaging (NBI) [3] is rapidly gaining broad acceptance. NBI offers a significantly more accurate delineation of the tumor by enhancing the contrast between mucosal surface and microvascular structures without use of dye [4]. Since the first report on diagnosis of NMIBC in 2008 [5], a growing body of literature demonstrates improvement in detection of NMIBC without additional morbidity or cost [3,6-8]. Regarding the therapeutic advantage of NBI during transurethral resection (TUR), the majority of research notes significant additional removal, particularly for CIS [4,9], small lesions [10], or extended margin of a large tumor [7]. As it is expected that it will eventually be determined from advanced identification, short-term studies on recurrence risk of NMIBC after application of NBI showed a significant decrease compared with conventional WLC [7,10-12].
However, at the same time, there is a lack of information regarding the accuracy of the surgeon’s identification of the suspicious lesion under NBI. Given the absence of universal guidelines on visual clues for NMIBC and reported low specificity of NBI compared with WLC, a decision based on NBI may result in increased false-positives, particularly for the patient with prior intravesical instillation. In addition, considering difference in diagnosis of urothelial carcinoma based on the nature of hematuria [13,14], a risk of unnecessary biopsy in an asymptomatic patient presenting with microscopic hematuria alone was a concern deterring the routine use of blue light cystoscopy [15]. Therefore, we evaluated its accuracy and efficacy in detection of the histologically proven malignancy for all abnormal lesions detected by NBI in common clinical scenarios involving suspicion of the presence of NMIBC with different risks, including microscopic hematuria, gross hematuria, and prior history of NMIBC.
From January 2012 to December 2013, 63 consecutive patients with prior NMIBC underwent cystoscopic evaluation, mainly for the presence of NMIBC, by a single urologist with over 10 years’ experience in management of bladder tumors for evaluation of microscopic hematuria (group I, n=20), gross hematuria (group II, n=19), and tumor recurrence (group III, n=24). Enhanced abdominal-pelvic computed tomography scan and urine cytology were routinely performed before cystoscopy. In the presence of pyuria at initial urinalysis, empirical antibiotics were administered until elimination before cystoscopy evaluation. In group III, bacillus Calmette–Guérin (BCG), Connaught strain, was administered as intravesical instillation for 6 weeks. This particular group was given further cystoscopic evaluations in our institution at 3 and 6 months after initial TUR for bladder cancer, then every 6 months up to 3 years, then annually. Cystoscopy was performed initially by WLC, then additional NBI. During cystoscopy, specimens for biopsy were taken from positive findings identified by WLC or NBI. In this series, considering reported superiority of cancer detection by NBI, particularly in sensitivity, all lesions that were abnormal in contrast with adjacent normal mucosa were defined as positive (Fig. 1). Random biopsies were performed for negative cystoscopy findings in the presence of positive cytology outcome. Histologic interpretations, including urine cytology were performed by a single uropathologist. After receiving approval from the local Institutional Review Board (YUH-14-0303-03), the collected data was analyzed to assess the accuracy of each cystoscopy modality. Diagnostic accuracy based on histologic outcome in each cystoscopy setting was assessed according to sensitivity, specificity, positive predictive value, negative predictive value, and accuracy. Sensitivity and specificity were then compared using the McNemar test. Two-sided null hypotheses of no difference were rejected if p-values were less than 0.05. All analyses were performed using IBM SPSS ver. 20.0(IBM Co., Armonk, NY).
Table 1 is a detailed summary of patient demographics. In urine cytology, 16 cases were abnormal, including 10 cases with atypical or suspicious cells. During cystoscopy, 40 abnormal cases were identified, including 22 cases (55%) by WLC and 18 additional cases (45%) by NBI only. Based on the results of urine cytology and cystoscopy, 66 cystoscopy biopsies from 47 patients were performed (including 18 random biopsies from 7 patients by cytology only). Multiple biopsies were performed in 13 cases (27.6%) with more than one suspicious finding on cystoscopy. Results of pathologic examination showed 17 cases of NMIBC (26.9%; 3 cases in group I, 8 cases in group II, and 6 cases in group III) from 21 sites (31.8%). The outcomes from random biopsies based only on cytology results were negative in all cases.
A summary of results from comparison of each cystoscopy setting is in Table 2. In per-case analysis, the overall sensitivity of additional NBI (100%) was similar to that of initial WLC (94.1%, p > 0.999), and the specificity of NBI (50%) was significantly lower than that of WLC (86.9%, p < 0.001). In subgroup analysis, the sensitivities and specificities of NBI and WLC were similar, except in group III, which had significantly lower specificity on NBI (38.9% vs. 88.9%, p=0.004) with similar sensitivity (100% [6 cases of NMIBC] vs. 83.3% [5 cases of NMIBC], p > 0.999). In per-biopsy analysis, the overall specificity was also significantly lower in NBI than in WLC (15.6% vs. 62.2%, p < 0.001), despite similarity in sensitivity (100% [21 sites of NMIBC] vs. 90.5% [19 sites of NMIBC], p=0.500). Decreased specificity of NBI was observed only in group III (10.5% vs. 78.9%, p < 0.001).
As a whole, based on identification by NBI only, 23 additional biopsies from 18 cases (5 cases in group I, 3 cases in group II, and 10 cases in group III) were performed for identification of one additional patient with NMIBC, who belonged to group III. In this particular group, to identify this specific patient, 15 additional biopsies were performed from 10 patients based on positive NBI findings (Table 3).
In group III, all patients had undergone prior intravesical instillation treatment with a median interval of 23 weeks (range, 4 to 420 weeks) from cystoscopy. None of the enrolled patients received maintenance BCG treatment over a year. In six patients with pathologically proven NMIBC, the interval from the last BCG instillation was similar (median, 24 weeks; range, 13 to 52 weeks; p=0.537). However, this interval was significantly shorter in cases with positive NBI findings than in those with negative NBI findings (median, 11.5 weeks vs. 51 weeks; p < 0.015). All seven patients who were NBI positive within 2 months after the last intravesical therapy were histologically proven as negative (Table 4).
The ideal endoscopic imaging modality should be highly sensitive for cancer detection, maintaining the ability to distinguish between benign and malignant lesions [16]. NBI cystoscopy is a plausible candidate, aimed at improving the detection of bladder tumors based solely on an optic technological advancement [9]. In short, NBI light is absorbed strongly by hemoglobin and penetrates only superficial layers of the tissue [6], highlighting the superficial and underlying vasculature [4]. Thereby, NBI improves visualization of tumors by enhancing the contrast between vascularized lesions and normal mucosa [10,16,17].
As positive evidence accumulates from early trials, particularly for advanced detection, enthusiasm regarding NBI is increasing. The missed-detection rate by conventional WLC is high, ranging from 10% to 20% [18]. In contrast, NBI allows more thorough primary tumor resection and reduces the number of missed tumors. In a recently updated meta-analysis for biopsy level analysis based on evidence from four studies of 1,195 lesions, the pooled sensitivity of NBI was 0.949; for WLC, it was only 0.751 (the pooled specificity was 0.548 in NBI; 0.719 in WLC) [3]. In addition, TUR performed using the NBI modality reduces the risk of recurrence of NMIBC by at least 10% at 1 year [12]. Several short-term randomized prospective studies demonstrated that NBI-assisted TUR could result in significant reduction of the residual tumor and recurrence rate [7,12]. In an effort to obtain more solid evidence, a large international randomized controlled trial is underway [19]. In comparison with 5-aminolevulinic acid (5-ALA) and hexaminolevulinate (HAM) cystoscopy, highly recommended for evaluation of NMIBC in contemporary guidelines [2], NBI has both economic and practical advantages, in that no preoperative preparation, including intravesical instillation, is required.
Nevertheless, because NBI is not cancer specific but only provides the morphologic aspect of suspicious lesions, the increased sensitivity and decreased specificity unfortunately results in increased false positives [20]. High false-positive rates may, in turn, lead to a substantial amount of unnecessary biopsy or resection on bladder tissue. In a prospective controlled trial conducted by Tatsugami et al. [4], among 161 abnormal sites from 104 consecutive patients with definite or suspected bladder cancer, 91 sites were identified by both NBI and WLC, while 70 sites were identified by NBI only. However, after histologic examination, only 55.7% (39/70) were malignant while 36.6% (59 sites) were proven false positive by NBI. Specificity for each modality was 70.9% for NBI and 86.2% for WLC. Similarly, Cauberg et al. [10] reported higher false-positivity of NBI (31.6%), compared with WLC (24.5%, p < 0.001), in 95 consecutive patients who were suspicious for bladder cancer. Four recent studies also reported a higher number of false-positives of 245 by NBI, in contrast to 115 by WLC [8].
These concerns for lower specificity and higher false positivity were reinforced in our series. In both per-case and per-site analysis, significantly lower overall specificity was observed for NBI compared with WLC and among 40 abnormalities identified by NBI, only 17 cases were proven malignant. Although NBI revealed 23 additional positive findings from 18 cases, only two additional NMIBC were found, in one patient. What is the cause of this phenomenon? One likely explanation is that many patients with recurrent disease had prior intravesical instillation, which causes erythematous change of the mucosa, raising the likelihood of a false-positive with NBI [21]. In a single center prospective study on 95 consecutive patients with suspicion of NMIBC, Cauberg et al. [10] reported that the false-positive rate in NBI was significantly higher (43.5% vs. 23.1%, p < 0.001) in cases with prior intravesical instillation, although this difference was not observed in WLC (34.5% vs. 19.0%, p=0.071). In a series on 50 patients conducted by four urologists with different experience, one experienced urologist considered more lesions positive for tumor than other urologists [22]. Therefore, individual specificity showed a wide range of 79 to 58 mainly because of prior BCG instillation. In our series, all patients in the follow-up group underwent prior BCG instillation, and it is noteworthy that the interval of cystoscopy from the last intravesical instillation was significantly shorter in cases with positive NBI findings. In all positive findings from NBI within 2 months after intravesical therapy, no malignancy was detected in histologic examination. In an effort to avoid the complexity induced by the post-BCG setting, some studies using photodynamic imaging included only a few patients treated with BCG, or excluded them altogether [23-25]. Currently, diagnosis by 5-ALA or HAM on NMIBC is not recommended within 3 months of BCG treatment. Nevertheless, contemporary guidelines emphasize the importance of maintenance BCG [2], which in turn particularly hinders the accuracy of NBI.
The authors well recognize the innate limitations of this study. First, because the decision on the biopsy was made by a single physician, other physicians might make a different judgment on the same lesions. In addition, because WLC was performed prior to NBI for the same patient, positive findings on WLC had an identical result in NBI cystoscopy, given the higher sensitivity of NBI. Second, while we focused on these three clinical scenarios requiring careful examination of the urinary tract, including cystoscopy for evaluation of the advantage of additional NBI over conventional WLC images, the risk of NMIBC may not be the same in each clinical group, requiring more cases, particularly for groups I and II. Third, although random biopsy was usually advised in the absence of definite cystoscopic findings concomitant with positive cytology, lower sensitivity in this clinical setting may decrease the efficacy of cystoscopy. All seven patients in this series had normal histologic findings. Finally, because there is no established criterion for diagnosing a positive lesion based upon NBI findings, we biopsied every abnormal lesion detected by NBI regardless of prior BCG. This policy may elevate the false-positive results, leading to decreased specificity. We believe that our results do not dissuade the urologist from performing NBI, because additional biopsy can be justified by the increased detection of NMIBC. Until agreement is reached on the judging standard of the image obtained by NBI, to avoid unnecessary biopsy we suggest that urologists keep in mind that not all abnormal lesions identified by NBI are malignant, particularly in the early period after intravesical BCG therapy. The outcome of ongoing trials may provide guidance for this practical question.
Despite a slight increase in additional detection, the specificity of NBI cystoscopy in identification of bladder urothelial carcinoma was significantly lower than that from conventional WLC. Particularly in evaluation for recurrence early after prior intravesical instillation using BCG, the decision based on NBI increased the use of unnecessary biopsy in the absence of an established standard for judging NBI findings.
References
1. Geavlete B, Stanescu F, Moldoveanu C, Jecu M, Adou L, Bulai C, et al. NBI cystoscopy and bipolar electrosurgery in NMIBC management: an overview of daily practice. J Med Life. 2013; 6:140–5.
2. Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013; 64:639–53.

3. Zheng C, Lv Y, Zhong Q, Wang R, Jiang Q. Narrow band imaging diagnosis of bladder cancer: systematic review and meta-analysis. BJU Int. 2012; 110(11 Pt B):E680–7.

4. Tatsugami K, Kuroiwa K, Kamoto T, Nishiyama H, Watanabe J, Ishikawa S, et al. Evaluation of narrow-band imaging as a complementary method for the detection of bladder cancer. J Endourol. 2010; 24:1807–11.

5. Bryan RT, Billingham LJ, Wallace DM. Narrow-band imaging flexible cystoscopy in the detection of recurrent urothelial cancer of the bladder. BJU Int. 2008; 101:702–5.

6. Naselli A, Introini C, Bertolotto F, Spina B, Puppo P. Narrow band imaging for detecting residual/recurrent cancerous tissue during second transurethral resection of newly diagnosed non-muscle-invasive high-grade bladder cancer. BJU Int. 2010; 105:208–11.

7. Geavlete B, Multescu R, Georgescu D, Stanescu F, Jecu M, Geavlete P. Narrow band imaging cystoscopy and bipolar plasma vaporization for large nonmuscle-invasive bladder tumors: results of a prospective, randomized comparison to the standard approach. Urology. 2012; 79:846–51.
8. Li K, Lin T, Fan X, Duan Y, Huang J. Diagnosis of narrowband imaging in non-muscle-invasive bladder cancer: a systematic review and meta-analysis. Int J Urol. 2013; 20:602–9.

9. Herr HW, Donat SM. A comparison of white-light cystoscopy and narrow-band imaging cystoscopy to detect bladder tumour recurrences. BJU Int. 2008; 102:1111–4.

10. Cauberg EC, Kloen S, Visser M, de la Rosette JJ, Babjuk M, Soukup V, et al. Narrow band imaging cystoscopy improves the detection of non-muscle-invasive bladder cancer. Urology. 2010; 76:658–63.

11. Naselli A, Introini C, Timossi L, Spina B, Fontana V, Pezzi R, et al. A randomized prospective trial to assess the impact of transurethral resection in narrow band imaging modality on non-muscle-invasive bladder cancer recurrence. Eur Urol. 2012; 61:908–13.

12. Herr HW, Donat SM. Reduced bladder tumour recurrence rate associated with narrow-band imaging surveillance cystoscopy. BJU Int. 2011; 107:396–8.

13. Patel JV, Chambers CV, Gomella LG. Hematuria: etiology and evaluation for the primary care physician. Can J Urol. 2008; 15 Suppl 1:54–61.
14. Messing EM, Young TB, Hunt VB, Gilchrist KW, Newton MA, Bram LL, et al. Comparison of bladder cancer outcome in men undergoing hematuria home screening versus those with standard clinical presentations. Urology. 1995; 45:387–96.

15. Davis R, Jones JS, Barocas DA, Castle EP, Lang EK, Leveillee RJ, et al. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol. 2012; 188(6 Suppl):2473–81.

16. Liu JJ, Droller MJ, Liao JC. New optical imaging technologies for bladder cancer: considerations and perspectives. J Urol. 2012; 188:361–8.

18. Jichlinski P, Leisinger HJ. Fluorescence cystoscopy in the management of bladder cancer: a help for the urologist! Urol Int. 2005; 74:97–101.

19. Naito S, van Rees Vellinga S, de la Rosette J. Global randomized narrow band imaging versus white light study in nonmuscle invasive bladder cancer: accession to the first milestone-enrollment of 600 patients. J Endourol. 2013; 27:1–3.
20. Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, et al. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004; 9:568–77.

21. Herr HW. Narrow-band imaging cystoscopy to evaluate the response to bacilli Calmette-Guerin therapy: preliminary results. BJU Int. 2010; 105:314–6.
22. Herr H, Donat M, Dalbagni G, Taylor J. Narrow-band imaging cystoscopy to evaluate bladder tumours: individual surgeon variability. BJU Int. 2010; 106:53–5.
23. Stenzl A, Burger M, Fradet Y, Mynderse LA, Soloway MS, Witjes JA, et al. Hexaminolevulinate guided fluorescence cystoscopy reduces recurrence in patients with nonmuscle invasive bladder cancer. J Urol. 2010; 184:1907–13.

24. Schumacher MC, Holmang S, Davidsson T, Friedrich B, Pedersen J, Wiklund NP. Transurethral resection of non-muscle-invasive bladder transitional cell cancers with or without 5-aminolevulinic acid under visible and fluorescent light: results of a prospective, randomised, multicentre study. Eur Urol. 2010; 57:293–9.

Fig. 1.
False-positive and true-positive cases using narrow band imaging (NBI) cystoscope. White light cystoscopy (WLC) (A; counted as negative) and NBI (B; counted as positive) cystoscopic finding of 64-year-old male who was priorly treated with transurethral resection of bladder for urothelial carcinoma of Ta, low grade. He finished his bacillus Calmette–Guérin (BCG) instillation 9 weeks before cystoscopy, and histologic examination revealed chronic inflammation. WLC (C; counted as positive) and NBI (D; counted as positive) cystoscopic finding of 53-year-old male who was treated with prior transurethral resection of bladder for urothelial carcinoma of T1, low grade. He finished his last BCG instillation 8 months prior, and histologic exanimation found Ta, papillary urothelial carcinoma with low malignant potential.
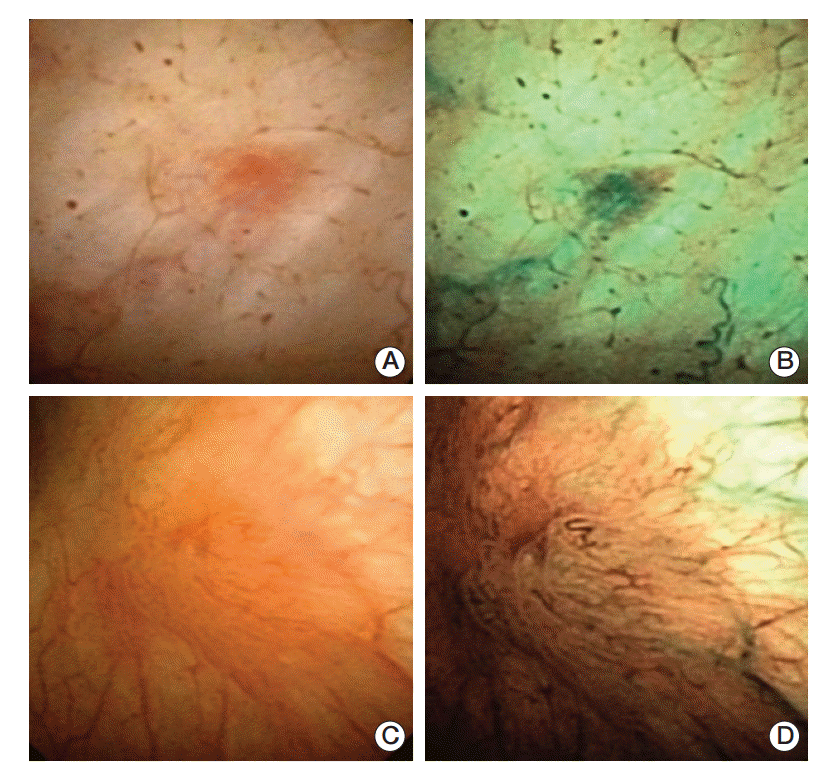
Table 1.
Patient demographics
| Demographics | Total (n=63) | Microscopic hematuria (n=20) | Gross hematuria (n=19) | Prior NMIBC (n=24) | p-valuea) |
|---|---|---|---|---|---|
| Median age (IQR, yr) | 66 (56-76) | 61 (53.7-65.2) | 75 (67-79) | 65.5 (53-76.7) | 0.007b) |
| Sex | |||||
| Male | 39 (61.9) | 11 (55) | 14 (73.7) | 14 (58.3) | 0.438 |
| Female | 24 (38.1) | 9 (45) | 5 (26.3) | 10 (41.7) | |
| Cytology outcome | |||||
| Normal | 47 (74.6) | 16 (80) | 11 (57.9) | 20 (83.3) | 0.348 |
| Atypical/suspicious | 10 (15.9) | 3 (15) | 5 (26.3) | 2 (8.3) | |
| Malignant cell | 6 (9.5) | 1 (5) | 3 (15.8) | 2 (8.3) | |
| Biopsy cases | |||||
| Done | 47 (74.6) | 13 (65) | 15 (78.9) | 19 (79.2) | 0.490 |
| Not done | 16 (25.4) | 7 (35) | 4 (21.1) | 5 (20.8) | |
| Biopsy sites | |||||
| Total number of biopsy core | 66 | 17 | 22 | 27 | |
| Single | 34 | 11 | 10 | 13 | |
| Multiple (over 2 cores) | 13 | 2 | 5 | 6 | |
| Histology outcome (per case) | |||||
| Benign | 46 (73.0) | 17 (85.0) | 11 (57.9) | 18 (75.0) | 0.156 |
| Malignancy | 17 (27.0) | 3 (15.0) | 8 (42.1) | 6 (25.0) | |
| Histology outcome (per biopsy) | |||||
| Benign | 45 (68.2) | 14 (82.4) | 12 (54.5) | 19 (70.4) | 0.172 |
| Malignancy | 21 (31.8) | 3 (17.6) | 10 (45.5) | 8 (29.6) | |
| NMIBC stage | |||||
| Ta | 7/17 (41.2) | 3/3 (100) | 3/8 (37.5) | 1/6 (16.7) | 0.126 |
| T1 | 9/17 (52.9) | - | 4/8 (50) | 5/6 (83.3) | |
| Over T2 | 1/17 (5.9) | - | 1/8 (12.5) | - | 0.623 |
| NMIBC grade | |||||
| PUNLMP | 7/16 (43.8) | 2/3 (66.7) | 2/7 (28.6) | 3/6 (50) | |
| Low grade | 5/16 (31.2) | 1/3 (33.3) | 2/7 (28.6) | 2/6 (33.3) | |
| High grade | 4/16 (25) | - | 3/7 (42.9) | 1/6 (16.7) | |
| WLC outcome | |||||
| Normal | 41 (65.1) | 14 (70) | 10 (52.6) | 17 (70.8) | 0.395 |
| Abnormal | 22 (34.9) | 6 (30) | 9 (47.4) | 7 (29.2) | |
| NBI outcome | |||||
| Normal | 23 (36.5) | 9 (45.0) | 7 (36.8) | 7 (29.2) | 0.554 |
| Abnormal | 40 (63.5) | 11 (55.0) | 12 (63.2) | 17 (70.8) | |
| Abnormal findings on cystoscopy | |||||
| Total cases | 40 | 11 | 12 | 17 | |
| Both WLC and NBI | 22 | 6 | 9 | 7 | |
| Only by WLC | - | - | - | - | |
| Only by NBI | 18 | 5 | 3 | 10 | |
Table 2.
Comparison by cystoscopy modality for detection of NMIBC
| Variable | Total | Microscopic hematuria | Gross hematuria | Prior NMIBC |
|---|---|---|---|---|
| WLC (per case) | ||||
| Sensitivity | 94.12 | 100 | 100 | 83.33 |
| Specificity | 86.96 | 82.35 | 90.91 | 88.89 |
| PPV | 72.73 | 50 | 88.89 | 71.43 |
| NPV | 97.56 | 100 | 100 | 94.12 |
| Accuracy | 88.89 | 85 | 94.74 | 87.5 |
| NBI (per case) | ||||
| Sensitivity | 100 | 100 | 100 | 100 |
| Specificity | 50*** | 52.94 | 63.64 | 38.89** |
| PPV | 42.50 | 27.27 | 66.67 | 35.29 |
| NPV | 100 | 100 | 100 | 100 |
| Accuracy | 63.49 | 60 | 78.95 | 54.17 |
| WLC (per biopsy) | ||||
| Sensitivity | 90.48 | 100 | 100 | 75 |
| Specificity | 62.22 | 50 | 50 | 78.95 |
| PPV | 52.78 | 30 | 62.50 | 60 |
| NPV | 93.33 | 100 | 100 | 88.24 |
| Accuracy | 71.21 | 58.82 | 72.73 | 77.78 |
| NBI (per biopsy) | ||||
| Sensitivity | 100 | 100 | 100 | 100 |
| Specificity | 15.56*** | 14.29 | 25 | 10.53*** |
| PPV | 35.59 | 20 | 52.63 | 32 |
| NPV | 100 | 100 | 100 | 100 |
| Accuracy | 42.42 | 29.41 | 59.09 | 37.04 |
Table 3.
Histologic outcome and cystoscopy modality in detection of NMIBC




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download