Abstract
Brain metastasis affects one third of patients with HER2-positive breast cancer after treatment with trastuzumab. Surgical resection and radiation therapy are often unsuccessful at accomplishing complete control of metastasis. Lapatinib is presumed to cross the blood-brain barrier, and exhibits clinical activities for treatment of HER2-positive breast cancer. A 43-year-old woman was treated for early breast carcinoma with total mastectomy, axillary lymph-node dissection, and adjuvant chemotherapy with cyclophosphamide plus doxorubicin. After the end of adjuvant trastuzumab therapy, she was diagnosed with panhypopituitarism due to pituitary metastasis. Surgical removal and whole brain radiation therapy were performed, but a portion of viable tumor remained. Only taking lapatinib, the size of the metastatic lesion began to shrink. Trastuzumab may have controlled the micro-metastasis of breast cancer, but it was unable to control its progression to the central nervous system. Lapatinib is a possible option for HER2-positive metastatic breast cancer patients with brain metastasis.
Overexpression of human epidermal growth factor receptor 2 (HER2) results in an aggressive form of breast cancer [1]; however, the introduction of trastuzumab has significantly improved the poor prognosis of this population of patients [2]. Although trastuzumab-based regimens are associated with improved control of HER2-positive metastatic breast cancer, one third of trastuzumab-treated patients still develop brain metastasis [3,4]. Trastuzumab is a large monoclonal antibody, therefore, the drug cannot cross the blood-brain barrier [5].
Pituitary metastasis is an unusual event of cancer progression, representing only 1% of pituitary lesions [6]. Approximately 30% of pituitary metastasis cases are from primary breast cancer, and less than 10% are symptomatic. Most common signs of pituitary metastasis are diabetes insipidus, hypopituitarism, visual difficulty, and headache [6,7]. There are multiple treatment modalities for pituitary metastasis. Surgical resection, however, is difficult due to tumor vascularity and local invasiveness [8], and radiation therapy is associated with substantial neurocognitive toxicity.
Lapatinib, an oral dual tyrosine kinase inhibitor of HER2 [9], has demonstrated activity in combination with capecitabine for treatment of HER2-positive metastatic breast cancer that progresses after treatment with trastuzumab-containing regimens [10-12].
Here, we report on the case of a patient with HER2-positive early breast cancer, who developed solitary pituitary metastasis after treatment with trastuzumab. After incomplete surgery and whole brain radiation therapy, the metastasis was controlled with lapatinib.
A 43-year-old woman presented with polydipsia, general weakness, gait disturbance, somnolence, and headache in September, 2012. She was diagnosed with a stage II breast cancer in April, 2011; a pathology report based on samples taken during total mastectomy with axillary lymph-node dissection showed invasive ductal carcinoma of pT2N0M0, which was estrogen receptor positive (moderate, 10%), progesterone receptor negative, and HER2-positive (2+ by immunohistochemistry and amplification index 5.0 by fluorescence in situ hybridization). Four cycles of adjuvant chemotherapy with cyclophosphamide 600 mg/m2 plus doxorubicin 60 mg/m2 were administered, followed by one year of adjuvant trastuzumab and daily tamoxifen therapy. The patient presented with the same symptoms—polydipsia, general weakness, gait disturbance, somnolence, and headache—1 month after completing the last cycle of adjuvant trastuzumab therapy.
Bitemporal hemianopsia was noted after her physical examination. No organomegaly or lymphadenopathy was found. As shown in Table 1, laboratory investigations were in line with the diagnosis of panhypopituitarism. Magnetic resonance imaging (MRI) of the patient’s brain showed an enhancing mass in the sellar and suprasellar regions (Fig. 1A). She started taking hormone replacement therapy (desmopressin acetate 50 μg twice a day, prednisolone 7.5 mg a day, in divided doses every morning and afternoon, and levothyroxine 75 μg a day).
Because tumor adhesion was suspected from the brain MRI, partial removal of the tumor was performed via transcranial approach. In the surgical field, the right optic nerve was squeezed by the tumor, compressed downward by the suprasellar main mass and upward by the stalk lesion. The tumor was fibrous and adhesive to surrounding vessels and nerves, therefore complete dissection of the margin was difficult. Pathologic reports indicated metastatic carcinoma, which clinically originated from the breast: estrogen receptor positive (30%), progesterone receptor negative, HER2-positive (3+ by immunohistochemistry) (Fig. 2). Whole brain radiation therapy (30 Gy in 12 fractions) was applied (Fig. 1B), followed by weekly paclitaxel chemotherapy, but the patient was unable to finish the second cycle of paclitaxel due to toxicity (grade 3 nausea, somnolence, and loss of memory).
Lapatinib (1,250 mg daily) plus capecitabine (2,000 mg/m2 on days 1 through 14 of a 21-day cycle) was started from January 2013. During the third cycle, the patient visited the emergency room with severe septic shock due to gastrointestinal toxicity of lapatinib and capecitabine combination regimen. This was an expected adverse event of the regimen. In addition to poor general condition, her serum sodium level changed rapidly despite treatment with desmopressin as panhypopituitarism originated from the metastatic brain lesion (Fig. 3). In addition, osmotic demyelination syndrome occurred, and her mentality changed from deep stupor to semi-comatose status for 2 months.
It took more than 3 months of rehabilitation for restoration of the patient’s neurocognitive function; chemotherapy was withheld during this period. Repeated MRIs showed a mild increase in the size of the enhancing lesion in the sella, suprasella, and the right basal ganglia (Fig. 1C). Because a 20% dose reduction of capecitabine still evoked adverse effects (general weakness and electrolyte imbalance), chemotherapy with lapatinib alone was started.
After 2 months of lapatinib monotherapy, the follow-up MRI showed a decrease in the size of the enhancing lesion (Fig. 1D). Repeated computed tomography of the patient’s chest, abdomen, and pelvis did not show recurrence of disease in any other extra-cranial locations. The patient continued to suffer from visual loss and hypothalamic dysfunctions, including hypothermia, weight gain, and loss of memory. However, by steadily taking lapatinib, she now maintains a daily activity of 1 to 2 Eastern Cooperative Oncology Group performance score.
This is a case involving early recurrence of breast cancer manifested with panhypopituitarism due to metastasis to the pituitary, pituitary stalk, and hypothalamus. The pituitary gland is a rare site of metastasis for all neoplasms (metastasis makes up less than 1% of pituitary tumors), and less than 10% of pituitary metastases are symptomatic [6,7]. Therefore, the clinical history of the patient shows a rare pattern of breast cancer metastasis.
Brain metastasis developed despite previous treatment with trastuzumab in early stage breast cancer. Recent studies have suggested that trastuzumab may not be active in the central nervous system [5], which could be a potential sanctuary site for disease progression in trastuzumab-treated patients with HER2-positive breast cancer. According to previous reports regarding pituitary metastasis from breast cancer, most patients are elderly, and have clinical and/or radiologic evidence of widespread disease, mainly to the lymph nodes, lung and bone, at the time they are diagnosed with pituitary metastasis [13,14]. In this case, trastuzumab may have controlled the micro-metastasis of breast cancer after the initial total mastectomy and lymph-node dissection, however, brain metastasis still occurred. Therefore, the patient experienced solitary central nervous system progression without evidence of extra-cranial lesions.
Surgery and radiation could not completely control the pituitary metastasis of the breast cancer. Metastatic pituitary lesions tend to be firm, diffuse, invasive, vascular, and hemorrhagic; total resection of tumor is difficult [6]. Tumor debulking is beneficial for alleviating local symptoms, especially visual field defect and headache, whereas symptoms such as diplopia and those related to pituitary failure remain unaffected [6]. The patient’s quality of life was significantly affected; she had been required to take hormone replacement therapy every day from the time of diagnosis with pituitary metastasis, and still suffers from loss of memory, hypothermia, weight gain, and complete visual loss.
Lapatinib is proven to be as active as first-line treatment of brain metastasis from HER2-positive breast cancer in combination with capecitabine [12]. It has low molecular weight, and therefore is assumed to be able to cross the blood-brain barrier [15]. Lapatinib and capecitabine treatment are sometimes associated with grade 3 and grade 4 toxicity (most commonly, diarrhea and hand-foot syndrome) [12], however, adjustment of regimen can overcome these issues.
Widespread use of trastuzumab for treatment of HER2-positive breast cancer leaves patients with an increased risk of brain metastasis [3,4]. The median survival period after central nervous system progression is about 13 months [3,4]. More follow-up is required; however, even 16 months after the initial diagnosis of brain metastasis, the patient’s brain lesion is well controlled with lapatinib monotherapy. Therefore, we suggest that lapatinib is beneficial for improving the survival and quality of life for patients with HER2-positive brain metastasis.
Pituitary metastasis is an uncommon but serious event of cancer progression because it can lead to pituitary insufficiency. Life-long hormone replacement therapy and deteriorated quality of life are inevitable. Lapatinib can be an effective alternative therapy for managing brain metastasis of HER2-positive breast cancer, in cases that cannot be completely controlled by surgery and/or radiation therapy.
References
1. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987; 235:177–82.
2. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001; 344:783–92.

3. Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003; 97:2972–7.

4. Stemmler HJ, Kahlert S, Siekiera W, Untch M, Heinrich B, Heinemann V. Characteristics of patients with brain metastases receiving trastuzumab for HER2 overexpressing metastatic breast cancer. Breast. 2006; 15:219–25.

6. Komninos J, Vlassopoulou V, Protopapa D, Korfias S, Kontogeorgos G, Sakas DE, et al. Tumors metastatic to the pituitary gland: case report and literature review. J Clin Endocrinol Metab. 2004; 89:574–80.

8. Gsponer J, De Tribolet N, Deruaz JP, Janzer R, Uske A, Mirimanoff RO, et al. Diagnosis, treatment, and outcome of pituitary tumors and other abnormal intrasellar masses. Retrospective analysis of 353 patients. Medicine (Baltimore). 1999; 78:236–69.

9. Rusnak DW, Affleck K, Cockerill SG, Stubberfield C, Harris R, Page M, et al. The characterization of novel, dual ErbB-2/EGFR, tyrosine kinase inhibitors: potential therapy for cancer. Cancer Res. 2001; 61:7196–203.
10. Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006; 355:2733–43.

11. Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008; 112:533–43.

12. Bachelot T, Romieu G, Campone M, Dieras V, Cropet C, Dalenc F, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013; 14:64–71.

13. Houck WA, Olson KB, Horton J. Clinical features of tumor metastasis to the pituitary. Cancer. 1970; 26:656–9.

14. Max MB, Deck MD, Rottenberg DA. Pituitary metastasis: incidence in cancer patients and clinical differentiation from pituitary adenoma. Neurology. 1981; 31:998–1002.
Fig. 1.
Magnetic resonance imaging of metastatic pituitary lesion. (A) At the time of diagnosis, a 35-mm strongly enhancing mass was observed in the sellar and suprasellar regions (September 2012). (B) Even after partial removal of the tumor via craniotomy and whole brain radiation therapy, viable tumor remained (27 mm, November 2012). (C) The size of the enhancing lesion had increased slightly (29 mm) 4 months after discontinuation of lapatinib and capecitabine due to gastrointestinal sepsis (June 2013). (D) After re-starting lapatinib monotherapy, the size of the enhancing mass decreased (25 mm, September 2013).
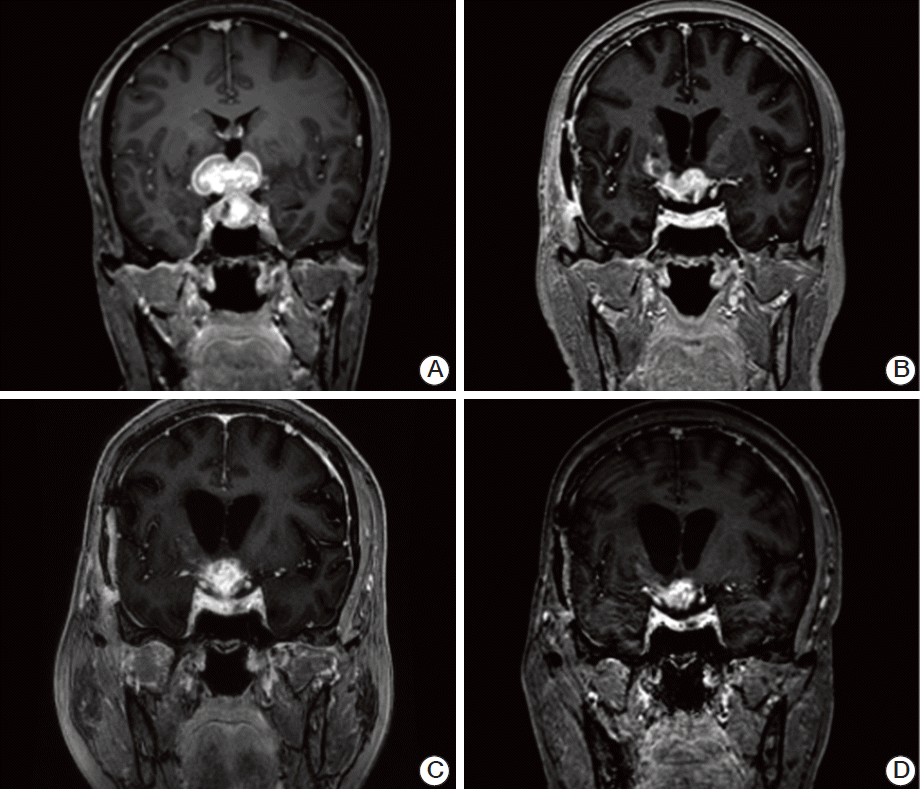
Fig. 2.
(A) Histology of invasive ductal carcinoma showing a predominantly trabecular pattern, high nuclear atypia, and high mitotic activity (H&E staining, ×100). (B) Histology of invasive ductal carcinoma metastasis to the brain, showing infiltration of malignant cells to the parenchyma (H&E staining, ×100).
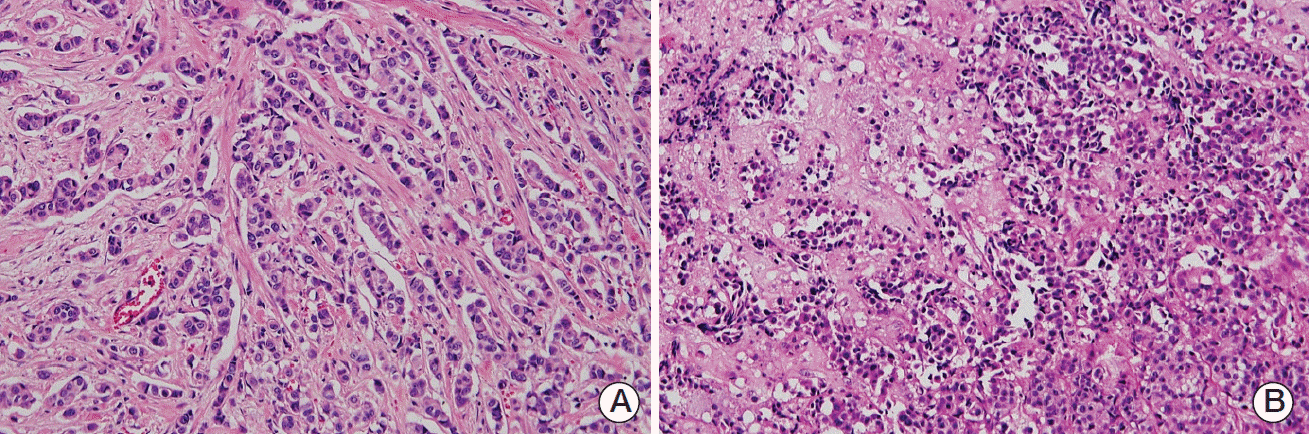
Fig. 3.
Due to gastrointestinal sepsis, the patient’s serum sodium level changed radically (March to April, 2013), and it was stabilized after the tumor was controlled by lapatinib (November 2013).
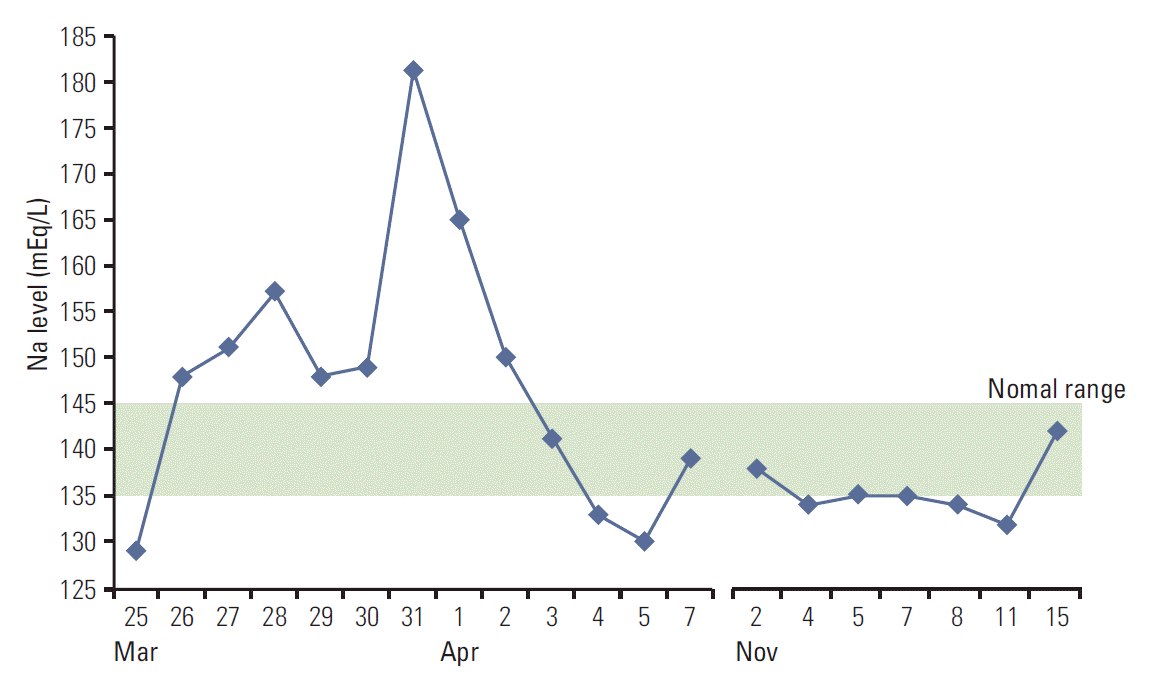
Table 1.
Blood and urine laboratory results




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download