Introduction
Materials and Methods
1. Literature search strategy
2. Eligibility criteria
3. Study selection and data extraction
Results
1. Literature search results
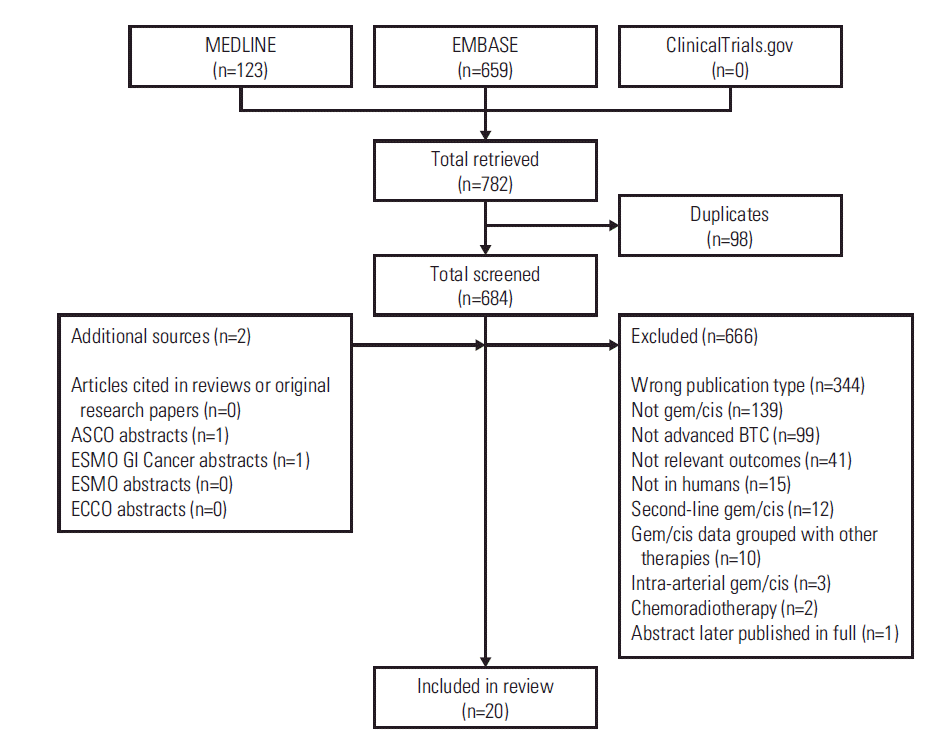 | Fig. 1.Publication flow diagram. ASCO, American Society of Clinical Oncology; BTC, biliary tract cancer; ECCO, European CanCer Organisation; ESMO, European Society for Medical Oncology; gem/cis, gemcitabine-cisplatin therapy; GI, gastrointestinal. |
Table 1.
| Publication |
Participants |
Main efficacy outcomes | ||||||
|---|---|---|---|---|---|---|---|---|
| No. | Median age (rangea), yr) | Male (%) | Primary tumor site (%) | Metastatic disease (%) | psb) (%) | Treatment regimen | ||
| Meta-analysis | ||||||||
| Mizuno et al. (2013) [37] (abstract), meta-analysis of ABC-02 (UK) and BT-22 (Japan) trials | 493 | 64 | ~50 | NR | NR | NR | NR; as per ABC-02 and BT22 | Hazard ratios for OS, PFS |
| - GC: 245 | (23-84) | |||||||
| - Gem: 248 | ||||||||
| Randomized controlled trials | ||||||||
| Kanget al. (2012) [27], South Korea | 96 | GC: 59 | GC: 63 | GC | 71 | GC | Gem: 1,000 mg/m2 | OS, PFS, RR |
| - GC: 49 | (32-77) | - GB: 27 | - PS0-1: 86 | (fixed 10 mg/m2/min) | ||||
| - S-l+Cis 47 | - Intrahepatic CAC: 41 | - PS2:14 | D1+D8 | |||||
| - Extrahepatic bile duct: 27 | Cis: 60 mg/m2Dl | |||||||
| - Ampulla: 6 | Cycle: 21 days | |||||||
| Median number of cycles (range): 6 (1-14) | ||||||||
| Okusaka et al. (2010) [32], Japan, BT-22 trial | 83c) | GC: 65.0 | GC: 43.9 | GC | NR | GC | Gem: 1,000 mg/m2 D1+D8 | OS, PFS, RR |
| - GC: 41 | (43-80) | - GB: 36.6 | - PS0: 82.9 | Cis: 25 mg/m2D1+D8 | ||||
| - Gem: 42 | - Extrahepatic bile duct: 19 | - PS1:17.1 | Cycle: 21 days | |||||
| - Intrahepatic bile duct: 34 | Median number of cycles (range): 6 (NR) | |||||||
| - Ampulla: 9.8 | ||||||||
| Valle et al. (2009) [16], UK, ABC-01 trial | 86 | GC: 63 | GC: 40.5 | GC | 61.9 | Kamofsky | Gem: 1,000 mg/m2 D1+D8 | PFS, RR, TTP |
| - GC: 42 | (38-76) | - Intrahepatic CAC: 28.6 | - PS100: 11.9 | Cis: 25 mg/m2D1+D8 | ||||
| - Gem: 44 | - Extrahepatic CAC: 21.4 | - PS90: 42.9 | Cycle: 21 days | |||||
| - GB: 23.8 | - CAC not otherwise specified: 23.8 | - PS80: 28.6 | Median number of cycles (range): 7.5 (1-8) | |||||
| - Ampulla: 2.4 | - PS70: 14.3 | |||||||
| - PS60: 2.4 | ||||||||
| Valle et al. (2010) [17], UK, ABC-02 trial (includes patients from ABC-01) | 410 | GC: 63.9 | GC: 47.1 | GC | 73 | GC | Gem: 1,000 mg/m2 D1+D8 | OS, PFS, RR |
| - GC: 204 | (32.8-81.9) | - GB: 35.8 | - PS0: 32.4 | Cis: 25 mg/m2D1+D8 | ||||
| - Gem: 206 | - Bile duct: 59.8 | - PS1: 54.4 | Cycle: 21 days | |||||
| - Ampulla: 4.4 | - PS2: 13.2 | Median number of cycles: NR | ||||||
| Median duration of treatment (GC): 21 weeks | ||||||||
| Prospective, nonrandomized studies | ||||||||
| Charoentum et al. (2013) [36] (abstract), Thailand | 34c) | 56 | 54 | NR | NR | PS0-1: 97 | Gem: 1,000 mg/m2 D1+D8 | PFS, RR |
| (34-66) | Cis: 75 mg/m2Dl | |||||||
| Cycle: 21 days | ||||||||
| Median number of cycles (range): 4 (3-6) | ||||||||
| Doval et al. (2004) [23], India | 30 | 53.5 | 27 | GB: 100 | 66 | Zubrod | Gem: 1,000 mg/m2 D1+D8 (RDI 95%) | OS, RR, TTP, response duration |
| - PS1: 73 | Cis: 70 mg/m2 D1 (RDI 99%) | |||||||
| - PS2: 27 | Cycle: 21 days | |||||||
| Median number of cycles (range): 4.5 (1-6) | ||||||||
| Giuliani et al. (2006) [25], Italy | 38 | 61 | 16 | GB: 26 | 53 | PS0-1: 92 | Gem: 1,000 mg/m2Dl+D8 | OS, RR, TTP; response duration |
| (40-75) | Bile duct: 74 | PS2: 8 | Cis: 75-80 mg/m2 (day NR) | |||||
| Cycle: 21 days | ||||||||
| Number of cycles: ≥ 3 | ||||||||
| Goldstein et al. (2011) [26], Australia/New Zealand | 50 | 58.7 | 46 | GB: 44 | 64 | PS0: 42 | Gem: 1,000 mg/m2 (fixed 10 mg/m2/min) | OS, PFS, RR, response duration |
| Intra- or extrahepatic bile duct: 50 | PS1: 46 | D1+D8 | ||||||
| Papilla of Vater: 4 | PS2: 12 | Cis: 20 mg/m2 D1+D8 | ||||||
| Unknown: 2 | Cycle: 21 days | |||||||
| Median number of cycles (range): 5 (1-21) | ||||||||
| Kim et al. (2006) [28], South Korea | 29 | 52 | 76 | GB: 34 | 59 | PS0: 7 | Gem: 1,250 mg/m2 | OS, RR, TTP, response duration |
| (37-69) | Intrahepatic CAC: 31 | PS1: 76 | D1+D8 (RDI 88%) | |||||
| Extrahepatic CAC: 31 | PS2: 17 | Cis: 60 mg/m2 D1 (RDI 91%) | ||||||
| Ampulla: 3 | Cycle: 21 days | |||||||
| Median number of cycles (range): 4 (1-9) | ||||||||
| Lee et al. (2006) [29], South Korea | 24 | 59 | 75 | GB: 0 | 71 | PS0-1: 79 | Gem: 1,000 mg/m2 | OS, RR, TTP |
| (45-71) | CAC: 100 | PS2: 21 | D1+D8 (RDI 77.8%) | |||||
| Cis: 70 mg/m2 D1 (RDI 78.6%) | ||||||||
| Cycle: 21 days | ||||||||
| Median number of cycles (range): 3 (2-6) | ||||||||
| Lee et al. (2008) [30], South Korea | 35c) | 60 | 66 | GB: 40.0 | 91 | PS0: 20.0 | Gem: 1,250 mg/m2 Dl+D8 | OS, RR, TTP, response duration, TTF |
| (36-68) | Intrahepatic bile duct: 51.4 | PS1: 71.4 | (RDI 84.5%) | |||||
| Extrahepatic bile duct: 5.7 | PS2: 8.6 | Cis: 70 mg/m2 D1+D8 | ||||||
| Papilla of Vater: 2.9 | (RDI 94.2%) | |||||||
| Cycle: 21 days | ||||||||
| Median number of cycles (range): 4 (1-8) | ||||||||
| Mahfouf et al. (2010) [20] | 143 | 57.1 | 37 | GB: 89.6d) | NR | PS0-1:100 | Gem: 1,250 mg/m2 Dl+D8 | OS, PFS, RR, disease-free survival |
| (abstract), Algeria | (32-75) | BTC: 10.4d) | Cis: 70 mg/m2 Dl | |||||
| Cycle: 21 days | ||||||||
| Median number of cycles (range): 4 (NR) | ||||||||
| Meyerhardt et al. (2008) [31], USA | 33 | 57 | 61 | GB: 15 | NR | PS0: 27 | Gem: 1,000 mg/m2 Dl+D8 | OS, PFS, RR, response duration |
| (42-73) | Intrahepatic CAC: 76 | PS1: 64 | Cis: 30 mg/m2 D1+D8 | |||||
| Extrahepatic CAC: 9 | PS2: 9 | Cycle: 21 days | ||||||
| Median number of cycles (range): 4 (1-21+) | ||||||||
| Park et al. (2006) [33], South Korea | 27 | Mean+SD: | 59 | GB: 48.1 | 81.5 | PS1: 81.5 | Gem: 1,000 mg/m2 Dl, D8, +D15 (RDI 86.7%) | OS, RR, response duration |
| 58.9±10.6 (28-77) | Intrahepatic bile duct: 33.3 | PS2: 18.5 | Cis: 75 mg/m2 Dl (RDI 95.5%) | |||||
| Extrahepatic bile duct: 18.6 | Cycle: 28 days | |||||||
| Median number of cycles (range): 5 (1-9) | ||||||||
| Singh et al. (2011) [21] | 10 | 55 | 40 | GB: 100 | NR | NR | Gem: 300 mg/m2 (for 6 hr) Dl +D8 | RR |
| (abstract), India | (33-67) | Cis: 70 mg/m2 D2 | ||||||
| Cycle: 21 days | ||||||||
| Median number of cycles (range): 3 (1-4) | ||||||||
| Thongprasert et al. (2005) [34], Thailand | 40c) | 50 | 58 | GB: 2.5 | NR | Median | Gem: 1,250 mg/m2 Dl + D8 | OS, RR, TTP, response duration |
| (31-69) | Intrahepatic CAC: 87.5 | Kamofsky | Cis: 75 mg/m2Dl | |||||
| Portahepatic CAC: 7.5 | PS: 80 (range, 60-90) | Cycle: 21 days | ||||||
| Ampulla: 2.5 | Median number of cycles: NR | |||||||
| Retrospective studiese) | ||||||||
| Charoentum et al. (2007) [22], Thailand | 42 | 51 | 67 | GB: 0 | 72 | PS0-1:83 | Gem: 1,250 mg/m2 Dl+D8 | OS, RR, TTP |
| CAC: 100 | PS2:17 | Cis: 75 mg/m2 Dl | ||||||
| Cycle: 21 days | ||||||||
| Median number of cycles (range): 4 (1-6) | ||||||||
| Eckmann et al. (2011) [24], USA | 85 | Mean±SD: | 57.6 | GB: 0 | Disseminated: NR | NR | OS, RR, response duration | |
| - GC: 53 | 61.0 ±11.5 | Intrahepatic CAC: 78.8 | 51.8 | |||||
| - Other: 32 | Hilar CAC: 21.2 | Multifocal: 27.0 | ||||||
| Wu et al. (2012) [35], Taiwan | 30 | 61.5 | 43% | GB: 13.3 | 70.0 | PS0-1: 86.7 | Gem: 1,000 mg/m2 Dl+D8 | OS, RR, TTP |
| (38-85) | Intrahepatic: 50.0 | PS2: 13.3 | Cis: 30 mg/m2 D1+D8 | |||||
| Extrahepatic: 10.0 | Cycle: 21 days | |||||||
| Ampulla: 20.0 | Median number of cycles (range): 3 (0.5-12) | |||||||
| Perihilar: 6.7 | ||||||||
GC, gemcitabine-cisplatin group; Gem, gemcitabine; NR, not reported; OS, overall survival; PFS, progression-free survival; Cis, cisplatin; GB, gallbladder; CAC, cholangiocarcinoma; PS, performance status; D, day; RR, response rates; TTP, time to progression; RDI, relative dose intensity; TTF, time to treatment failure; SD, standard deviation; BTC, biliary tract cancer.
2. Overview of study characteristics
3. Efficacy outcomes
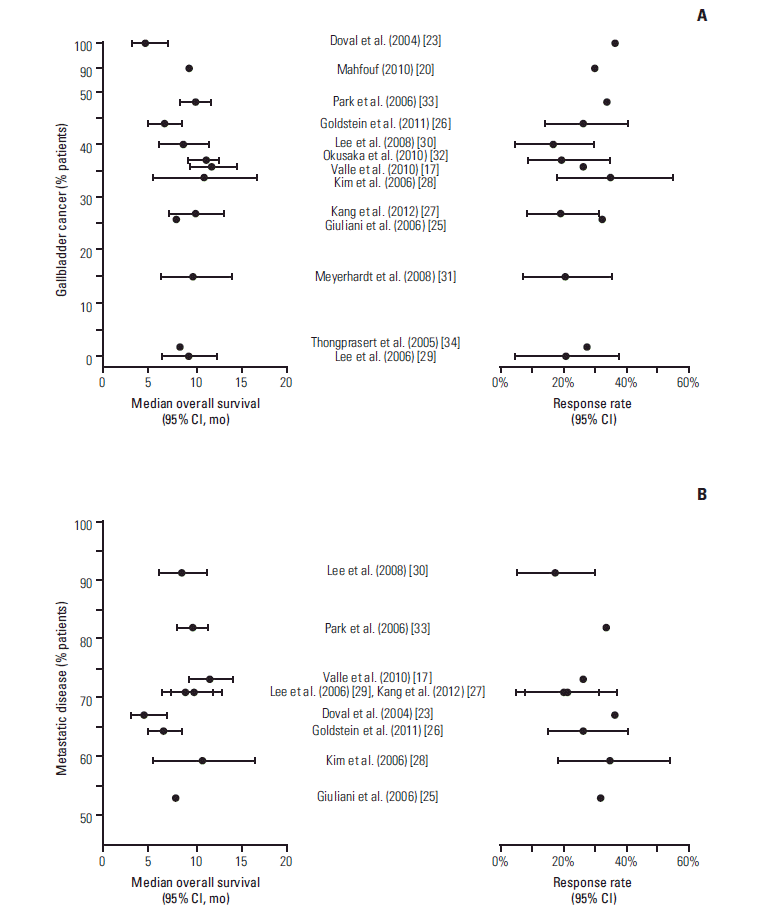
1) Overall
 | Fig. 2.Forest plots of median overall survival (A) and overall response rate (B) reported in individual publications of prospective studies. Error bars represent 95% confidence intervals (CI; where reported). The number of participants treated with gemcitabine-cisplatin in each study is shown in parentheses. |
Table 2.
| Publication | Median OS (95% CIa), mo) | Median PFS (95% CIa), mo) |
Response ratesb) (95% CIa),%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (CR+PR) | DCR (CR+PR+SD) | CR | PR | SD | PD | NE | |||
| Randomized controlled trials | |||||||||
| Kang et al. (2012) [27] | 10.1 (7.1-13.1) | 5.7 (3.6-7.7) | 19.6 (8.1-31.1) | 71.7 (58.7-84.7) | 4.3 | 15.2 | 52.2 | 28.3 | NA |
| Okusaka et al. (2010) [32], BT-22 trial | 11.2 (9.1-12.5) | 5.8 (4.1-8.2) | 19.5 (8.8-34.9) | 68.3 (51.9-81.9) | 0 | 19.5 | 48.8 | 22.0 | 9.8 |
| Valle et al. (2009) [16], ABC-01 trial | NR | 6-month PFS: 57.1% (41.0-70.3) | 27.8 | 75.0 | 0 | 27.8 | 47.2 | 25.0 | NA |
| Valle et al. (2010) [17], ABC-02 trial (includes patients from ABC-01) | 11.7 (9.5-14.3) | 8.0 (6.6-8.6) | 26.1 | 81.4 | 0.6 | 25.5 | 55.3 | 18.6 | NA |
| Prospective, nonrandomized studies | |||||||||
| Charoentum et al. (2013) [36] (abstract) | NR | 6 (range, 3-13) | 32.4c) | 76.5c) | 0 | 32.4c) | 44.1c) | 23.5c) | NA |
| Doval et al. (2004) [23] | 4.6d) (3.2-7.1) | NR | 36.6c) | 60.0c) | 13.3 | 23.3 | 23.3 | 13.2 | 27 |
| Giuliani et al. (2006) [25] | 8+ (range, 2-15) | NR | 32 | 53 | 3 | 29 | 21 | 47 | NA |
| Goldstein et al. (2011) [26] | 6.8 (5.0-8.7) | 4 (2.5-6.8) | 26 (14.6-40.4) | 50 | 0 | 26 | 24 | 44 | 6 |
| Kim et al. (2006) [28] | 11.0 (5.49-16.5) | NR | 34.5 | 48.3c) | 0 | 34.5 (17.9-54.3) | 13.8 | 44.8 | 6.9 |
| Lee et al. (2006) [29] | 9.30 (6.43-12.17) | NR | 20.8 | 70.8c) | 0 | 20.8 (4.5-37.0) | 50.0 (29.9-70.0) | 29.2 (11.0-47.3) | NA |
| Lee et al. (2008) [30] | 8.6 (6.1-10.4) | NR | 17.14 (4.7-29.6) | 45.7c) | 0 | 17.1 | 28.6 | 45.7 | 8.6 |
| Mahfouf (2010) [20] (abstract) | 9.3 | 4.7 | 30 | 52.4c) | 6.3c) | 23.8c) | 22.4c) | 47.6c) | NA |
| Meyerhardt et al. (2008) [31] | 9.7 (6.4-13.8) | 6.3 (4.8-14.9) | 21c) | 57.6c) | 0 | 21 (7-35) | 36 (20-52) | NR | NA |
| Park et al. (2006) [33] | 10.0 (8.4-11.6) | NR | 33.3 | 59.3c) | 0 | 33.3 | 25.9 | 40.7 | NA |
| Thongprasert et al. (2005) [34] | 8.3 (range, 0.8-21.9)d) | NR | 27.5 | 60c) | 0 | 27.5 | 32.5 | 40 | NA |
For comparative studies, data shown are for the gemcitabine-cisplatin group only. OS, overall survival; CI, confidence interval; PFS, progression-free survival; CR, complete response; PR, partial response; DCR, disease control rate; SD, stable disease; PD, progressive disease; NE, not evaluable; NA, not applicable; NR, not reported.
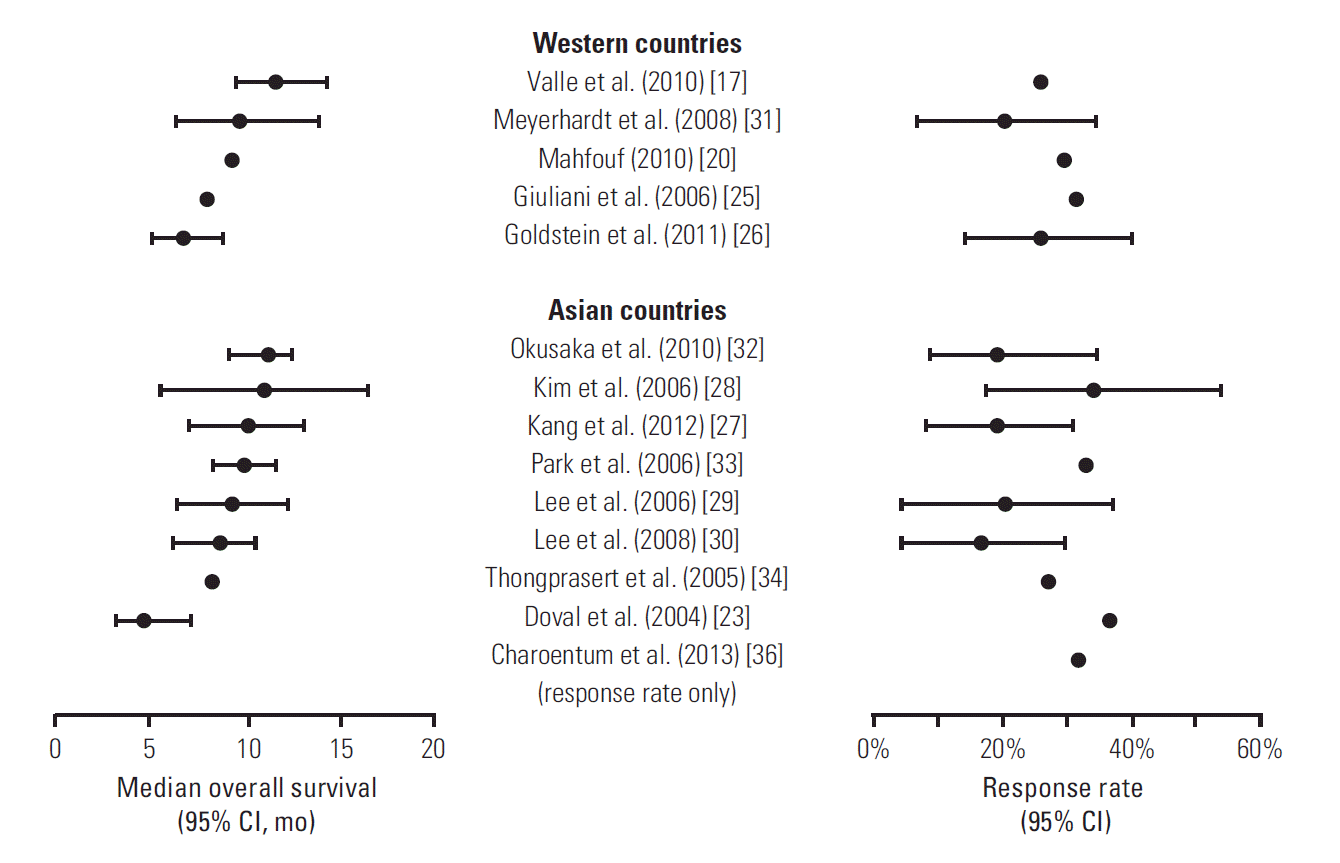
2) Exploratory subgroup analyses
4. Safety outcomes
1) Overall
Table 3.
| Publication |
Incidence of grade 3/4 toxicities (%) |
Treatment-related deaths/discontinuations | ||||||
|---|---|---|---|---|---|---|---|---|
| Anemia | Neutropenia | Thrombocytopenia | Vomiting | Nausea | Fatigue | Other (≥ 5% of participants) | ||
| Randomized controlled trials | ||||||||
| Kang et al. (2012) [27] | 22.4 | 49.0 | 22.4 | 4.1 | 4.1 | 4.1 | Leukopenia: 24.4 | No treatment-related deaths or discontinuations |
| (asthenia) | Neuropathy: 6.8 | |||||||
| Okusaka et al. (2010) [32], BT-22 trial | 34.1a) | 56.1 | 39.0 | 0 | 0 | NR | Leukopeniab): 29.3 | No treatment-related deaths |
| Hemoglobin decreased: 36.6 | ||||||||
| AST increased: 17.1 | ||||||||
| ALT increased: 24.4 | ||||||||
| GGT increased: 29.3 | ||||||||
| ALP increased: 7.3 | ||||||||
| Blood sodium decreased: 17.1 | ||||||||
| Valle et al. (2009) [16], ABC-01 trial | 2.4 | 14.3 | 11.9 | 7.1 | 0 | 28.6 | Infection (non-neutropenic): 19.0 | 3 Treatment-related discontinuations |
| Bilirubin: 11.9 | ||||||||
| Transaminases: 11.9 | ||||||||
| Valle et al. (2010) [17], ABC-02 trial (includes patients from ABC-01) | NR | 25.3 | 8.6 | 5.1 | 4.0 | 18.7 | Leukopeniab):15.7 | 1 Death possibly treatment-related |
| Hemoglobin decreased: 7.6 | ||||||||
| ALT increased: 9.6 | 17 Treatment-related discontinuations (of 162 ABC-02 patients only) | |||||||
| Other abnormal liver function: 13.1 | ||||||||
| Any abnormal liver function: 16.7 | ||||||||
| Infection without neutropenia: 6.1 | ||||||||
| Infection with neutropenia: 10.1 | ||||||||
| Any infection: 18.2 | ||||||||
| Prospective, nonrandomized studies | ||||||||
| Charoentum et al. (2013) [36] (abstract) | 11 (Gr 3) | 6 (Gr 4) | NR | NR | NR | NR | None | No treatment-related deaths |
| Doval et al. (2004) [23] | 36 | 34c) | 17 | 30 | NR | Leukopenia: 17 | 2 Treatment-related deaths | |
| (combined) | Hepatic: 10 | |||||||
| Renal: 6 | ||||||||
| Giuliani et al. (2006) [25] | 11 | 34 | 14 | 0 | NR | NR | None | No treatment-related deaths or discontinuations |
| Goldstein et al. (2011) [26] | 20 | 40 | 24 | 8 | 6 | 16 | Neutropenic fever: 8 | 1 Treatment-related death |
| Infection with normal | 16% Treatment-related discontinuation | |||||||
| neutrophils: 18 | ||||||||
| Kim et al. (2006) [28] | 3.4 | 13.8 | NR | 3.4 | 3.4 | NR | Neutropenic fever: 7.0 | No treatment-related deaths |
| Lee et al. (2006) [29] | 8.5 | 12.5 | 12.5 | 0 | 0 | NR | Leukopenia: 12.5 | 1 Treatment-related death |
| Diarrhea: 5 | ||||||||
| Lee et al. (2008) [30] | 6.8d) | 35.8d) | 17.6d) | 2.7d) | 3.4d) | NR | None | NR |
| Mahfouf 2010 [20] (abstract) | 5 | 2 | 1 | 20 | NR | 2 | NR | NR |
| Meyerhardt et al. (2008) [31] | 20 | 33 | 23 | 13 | 20 | 10 | Any toxicity: 70 | 33% Treatment-related discontinuation |
| 19% Withdrew consent due to toxicity | ||||||||
| Park et al. (2006) [33] | 29.6 | NR | 22.2 | 18.5 (combined) | NR | Leukopenia: 25.9 | No treatment-related deaths | |
| Singh et al. (2011) [21] (abstract) | 20 (combined)e) | 100 (combined)f) | NR | Alopecia: 40f) | NR | |||
| Thongprasert et al. (2005) [34] | 4.33 | 1.73 | 2.97 | 0 | 0 | NR | No other Gr3/4 | NR |
| Retrospective studies | ||||||||
| Charoentum et al. (2007) [22] | 33 | 21 | 5 | 0 | 0 | NR | None | NR |
| Eckmann et al. (2011) [24] | 1.9g) | 1.9g) | 5.7g) | NR | NR | 3.8g) | Increased creatinine: 11.3g) | 30% Treatment-related discontinuations |
| Wu et al. (2012) [35] | 16.7 | 13.3 | 0 | 0 | 0 | NR | Infection: 26.7 | |
| ALT increased: 6.7 | NR | |||||||
For comparative studies, data shown are for the gemcitabine-cisplatin group only. Where reported separately, grade 3 and 4 toxicities were added for inclusion in this table. NR, not reported; AST, aspartate transaminase; ALT, alanine transaminase; GGT, gamma-glutamyl transpeptidase; ALP, alkaline phosphatase; Gr, grade.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download