Abstract
Purpose
OPB-31121 is an oral STAT3 inhibitor with a good preclinical antitumor activity. This phase I dose-escalation study of OPB-31121 was conducted to determine maximum-tolerated dose (MTD), safety, pharmacokinetics, and preliminary antitumor efficacy in patients with advanced solid tumors.
Materials and Methods
Patients received OPB-31121 once daily for 28 days of each cycle followed by 2 weeks rest. A standard 3+3 design was used for dose-escalation. Safety and response were evaluated by the National Cancer Institute–Common Terminology Criteria for Adverse Events (NCI-CTCAE) ver. 3.0 and Response Evaluation Criteria in Solid Tumor (RECIST) ver. 1.0, respectively.
Results
Twenty-five patients were treated with OPB-31121 at five dose levels: 100 mg (n=4), 200 mg (n=3), 400 mg (n=3), 600 mg (n=7), and 800 mg (n=8). Seven patients discontinued treatment during cycle 1 for various reasons other than study drug-related adverse events. Among 18 patients who were evaluable for dose-limiting toxicity (DLT), three DLTs were observed: one DLT (grade 3 vomiting) at 600 mg and two DLTs (grade 3 vomiting, grade 3 diarrhea) at 800 mg. The MTD was determined as 800 mg/day. Common adverse events were gastrointestinal adverse event including nausea (84%), vomiting (80%), and diarrhea (72%). Pharmacokinetics did not demonstrate dose-proportionality of OPB-31121. Eight patients had stable disease and 10 patients had disease progression. Two patients (1 colon cancer, 1 rectal cancer) showed tumor shrinkage. One gastric cancer patient continued treatment up to cycle 13 before disease progression.
Signal transducer and activator of transcription (STAT) family proteins are latent cytoplasmic transcription factors that are activated in response to various stimuli, such as cytokines (e.g., interleukin 6), growth factors (e.g., epidermal growth factor, transforming growth factor α, hepatocyte growth factor) and hormones, and convey signals to the nucleus [1-4]. Among seven members of this family, aberrant STAT3 activity is known to be involved in all stages of tumor development [5-7]. In addition, many proto-oncogenes also require STAT3 for their oncogenic functions [8,9]. The effect of activated STAT3 is not only limited to tumor development and progression but also influences the outcome of chemotherapy and/or radiotherapy [10,11]. This critical role of STAT3 in the molecular pathogenesis of many tumors provides promise for targeting this protein for discovery of useful new anticancer drugs [12]. However, no specific inhibitor of STAT3 itself with drug-like characteristics has been introduced into clinical practice so far. Currently the most promising compound is JAK2 inhibitor, which targets upstream of STAT3 [13].
OPB-31121 is a novel low-molecular-weight compound from a compound library of antifibrotic agents. This compound is orally available. In our previous preclinical study, OPB-31121 showed a potent growth inhibition effect against gastric cancer cells and it also showed synergistic activity in combination with cytotoxic chemotherapeutic agents [14]. OPB-31121 is unique in that it has no appreciable effect on the activity of kinases or receptors as well-known target molecules for cancer therapy. Modulation of STAT3 is a key molecular action mechanism for the antitumor effects of OPB-31121, which shows promise as a useful new anticancer agent.
These promising preclinical data led to this phase I, firstin-human, dose-escalation study of OPB-31121 in patients with advanced solid tumors. The primary objective was to determine the maximum-tolerated dose (MTD) of OPB-31121 when administered once daily for 28 days. Secondary objectives included assessment of safety and tolerability, doselimiting toxicity (DLT), preliminary antitumor activity, and characterization of the pharmacokinetics.
This was a phase I, first-in-human, open-label, non-randomized, single-center, dose-escalation study of OPB-31121 in patients with advanced solid tumors. This study was approved by the Institutional Review Board of Seoul National University Hospital and was registered with the US National Library of Medicine (ClinicalTrials.gov) as NCT00657176.
The following inclusion criteria were used for patient selection: (1) histologically confirmed solid tumors refractory to standard therapy or for which there is no standard therapy; (2) age ≥ 19 years; (3) the Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2; (4) life expectancy of longer than three months; (5) adequate organ function (absolute neutrophil count ≥ 1,500/μL, platelet count ≥ 75,000 cells/μL, hemoglobin ≥ 10.0 g/dL, serum creatinine ≤ 1.5×upper limit of normal [ULN], serum bilirubin ≤ 2.5×ULN, aspartate aminotransferase, alanine transaminase, alkaline phosphatase ≤ 2.5×ULN); and (6) capable of swallowing OPB-31121 tablets. Written informed consent was obtained from all patients. The important exclusion criteria were (1) symptomatic central nervous system metastasis; (2) prior chemotherapy, radiation therapy, or surgery within 4 weeks prior to enrolling in the study; (3) uncontrolled concurrent illness, including active infection, heart failure, angina pectoris, and cardiac arrhythmia; and (4) use of CYP3A4 and CYP2C9 inducers, inhibitors, or substrates, and CYP2B6, CYP2C8, and CYP2D6 substrates.
OPB-31121 was administered orally to patients after breakfast once daily for 28 days followed by 2 weeks rest in each cycle of treatment. In the first cycle (cycle 1), OPB-31121 was administered on day 1, followed by a 2-day treatment-free interval for pharmacokinetics evaluation, and then administration resumed on day 4 and continued until day 28. The starting dose was 100 mg/day, and dose was escalated using 3+3 design. In each cohort, patients who were withdrawn for any reason other than DLT before completion of the 28-day administration period were replaced by other patients.
For management of nausea and vomiting, metoclopramide, 5-HT3 antagonist, NK1 receptor antagonist or dexamethasone were allowed.
Patients in whom antitumor effect was assessed as ‘stable disease’ (SD), ‘partial response’ (PR), or ‘complete response’ (CR) for overall response at the end of cycle 1 of treatment were allowed to receive cycle 2 of treatment after a 2-week rest period. Treatment was to be continued until the subject experienced disease progression or unacceptable toxicity, withdrew consent, or required treatment with another therapeutic modality.
DLT was defined as follows: (1) grade 4 neutropenia lasting for ≥ 7 days, grade 3 or 4 neutropenia with fever or infection; (2) grade 4 thrombocytopenia or grade 3 thrombocytopenia lasting for ≥ 7 days; (3) grade 3 or 4 nausea/vomiting or diarrhea despite optimal use of antiemetic drugs or antidiarrheal drugs; (4) other grade 3 non-hematological toxicity (except alopecia); (5) missed doses: more than three consecutive doses per cycle due to treatment related toxicity;and (6) delay of > 2 weeks in administration of the next cycle due to inadequate recovery from toxicity of cycle 1.
The lowest dose at which DLT was observed in 2 or more of the three or six patients in cycle 1 of treatment was judged to be the MTD.
Blood was collected before and 1, 2, 4, 6, 8, 12, 24 (days 2 and 29), 36 (days 2 and 29), 48 (days 3 and 30), 60 (days 3 and 30), and 72 (days 4 and 31) hours after dosing. Plasma concentrations of OPB-31121 were measured by liquid chromatography tandem mass spectrometry (LC-MS/MS) in accordance with the specified procedure of the bioanalytical laboratory.
The primary outcomes were safety and tolerability, which were measured by adverse events (AEs), vital sign, body weight, electrocardiogram, and laboratory tests. Safety was assessed according to the National Cancer Institute–Common Terminology Criteria for Adverse Events (NCI-CTCAE) ver. 3.0. The secondary outcomes were pharmacokinetics and efficacy. The following pharmacokinetic parameters were determined: maximum (peak) plasma concentration (Cmax), time to Cmax (tmax), area under concentration-time curve (AUC), terminal-phase elimination half-life (t1/2,z), CL/F and accumulation ratio. Efficacy was measured using Response Evaluation Criteria in Solid Tumor (RECIST) ver. 1.0. Tumor responses were assessed after cycle 1 or earlier in patients with suspected progression.
A total of 25 patients were enrolled in the study and received the investigational product. Baseline characteristics of patients are shown in Table 1. Median age was 53 years (range, 19 to 76 years). Sixteen patients (64.0%) were male. Six patients had ECOG performance status 0, 18 ECOG 1, and one ECOG 2. The most common tumors were colorectal cancer [8] and gastric cancer [6]. Others included lung cancer, esophageal cancer, melanoma, breast cancer, and pancreatic cancer, etc. All patients had been heavily pretreated with multiple lines of chemotherapy before enrollment.
The investigational products were administered at doses of up to 800 mg/day. Finally, 18 patients completed cycle 1 of the study, and seven patients (1 patient at 100 mg level, 1 patient at 600 mg level, and 5 patients at 800 mg level) discontinued during cycle 1 for various reasons (consent withdrawal, 4; disease progression, 1; other medical illness, 2). The reasons for consent withdrawal were aspiration pneumonia in one patient, dyspnea in one patient, and refusal of further treatment in two patients (Table 2).
DLT was reported in three patients. Two patients (1 patient at 600 mg, 1 patient at 800 mg) experienced grade 3 vomiting and one patient at 800 mg experienced grade 3 diarrhea. Thus, the MTD of OPB-31121 was determined as 800 mg/day once daily.
Most commonly reported AEs were nausea (84%), vomiting (72%), diarrhea (68%), and asthenia (56%). Most were grade 1/2 except DLT cases (Table 3). Grade 1/2 anorexia and asthenia were 40% and 56%. Grade 1/2 anemia, neutropenia, and thrombocytopenia were reported as 12%, 4%, and 4%, respectively. Death occurred in two patients, at 600 mg (progression of underlying disease, timing of death was 51 days after last medication of OPB-31121) and 800 mg (pulmonary embolism, on the last day of medication of OPB-31121), respectively.
The maximum plasma concentration of OPB-31121 was reached between 2.0 and 6.0 hours after single and multiple administrations at all dose levels (Table 4). After that, OPB-31121 was gradually eliminated from the plasma with t1/2, z of 17.92 to 44.30 hours on day 1, and 24.80 to 66.60 hours on day 28, and the plasma concentration of OPB-31121 could still be measured 72 hours after administration at all dose levels. Higher exposure was observed on day 28 compared to day 1 (Fig. 1). No dose-proportionality for OPB-31121 was confirmed.
Tumor response was assessed in 18 patients. There was no patient with CR or PR response. Overall responses were assessed as SD in eight patients and progressive disease in 10 patients (Table 5). Two patients obtained tumor shrinkage (1 colon cancer and 1 rectal cancer, –8.5% and –3.3%, respectively). One patient with gastric cancer at 400 mg continued the study treatment up to cycle 13 before disease progression.
One patient with gastric cancer at 400 mg continued the study treatment up to cycle 13 before disease progression. One patient with colon cancer at 600 mg continued the study up to cycle 4. One patient with non-small cell lung cancer at 100 mg continued the study up to cycle 3. Three patients (one gastric cancer, one hepatocellular carcinoma, and one rectal cancer) continued the treatment up to cycle 2.
This was the first-in-human phase I study of the novel STAT3 inhibitor, OPB-31121, once daily for 28 days in patients with advanced solid tumors, and information on the safety, tolerability, pharmacokinetics and preliminary efficacy was provided. The results of this study provide evidence of the feasibility of inhibition of STAT3 in patients with solid tumor. The MTD of oral OPB-31121 administered on a continuous daily schedule was defined as 800 mg/day on the basis of DLTs of vomiting and diarrhea. Up to the MTD, most common AEs were nausea (84%, all grade), vomiting (80%, all grade), and diarrhea (72%, all grade). Therefore, more careful attention regarding gastrointestinal AEs and their active management is advised in further clinical development of OPB-31121.
The pharmacokinetics of OPB-31121 in plasma was confirmed in the study. Dose-proportionality of OPB-31121 was not found. The exposures (i.e., AUC and Cmax) of OPB-31121 had large deviations. The reasons for these deviations may be as follows. (1) Due to the physical properties of OPB-31121: OPB-31121 is practically insoluble under neutral pH conditions (solubility: 2.38×10-6 % w/v at pH 7). Therefore, OPB-31121 may be deposited in the intestine. (2)Due to a multidrug transporter mechanism: since OPB-31121 is an MDR-1 substrate, OPB-31121 would be excreted by P-glycoprotein (P-gp) in the intestine. It is known that the expression of P-gp in intestine varies among individuals. Therefore, the pharmacokinetics of OPB-31121 in plasma may be affected by variations in P-gp expression. (3) Due to the sensitivity of OPB-31121 detection: when the plasma concentration decreased to below the lower limit of quantification relatively rapidly after tmax in a subject, there were very few evaluable time points for the subject. In this case, the values of AUC∞ may have been underestimated.
After initiation of our study, another phase I study using OPB-31121 was conducted to test a different schedule [15]. That study tested twice daily administration of OPB-31121 for 21 days of each 28-day cycle, and enrolled 30 patients with solid tumor and tested six dosing levels. The DLTs were grade 3 vomiting, grade 3 diarrhea, and grade 3 lactic acidosis, which leads to MTD of 300 mg. Therefore, both studies of OPB-31121 showed the same DLTs despite different dosing schedules.
The role of STAT3 in cancer is indicated by numerous avenues of evidence and there are several strategies to block STAT3 and its signaling pathways; inhibition of ligand, inhibition of kinases that phosphorylate the receptor, induction of the activity of phosphatases which dephosphorylate STAT3, inhibition of upstream JAK kinase, blocking the translocation from cytoplasm to nucleus, direct inhibition of STAT3 DNA binding and transcriptional activity, and inhibition of STAT3 activity by STAT3 antisense. Compounds which block STAT3 have been shown to have off target effects and this effect limited the usefulness of some of these compounds. JAK2 inhibitor is currently the most promising compound [13]. The main reason for the success of JAK2 inhibitor in myelofibrosis lies in an activating point mutation in the JAK2 gene observed in approximately 96%, 50%, and 50% of patients with polycythemia vera, essential thrombocythemia, and primary myelofibrosis, respectively [16-19]. However, in solid tumors, mutations in a critical functional domain of JAK genes are rarely or not involved in the frequent JAK/STAT pathway activation [20].
Compared to previous compounds targeting STAT3, OPB-31121 has a unique action mechanism of modulating STAT3. In this study, patients with colon cancer and rectal cancer showed tumor shrinkage, which is a consistent finding of in vivo study showing good antitumor activity in colon cancer. These results are supported by reports on the importance of the STAT signaling pathway in colorectal cancer [21,22]. STAT3 activation in gastric cancer has been repeatedly reported [23-25]. In our study, one gastric cancer patient achieved 19 months of progression-free survival. To the best of our knowledge, this is the first report on the antitumor effects of STAT3 inhibition in colon cancer and gastric cancer patients. However, finding a good biomarker to STAT3 inhibitor is still a challenge.
In conclusion, this study demonstrates feasibility of STAT3 inhibition in patients with advanced solid tumor. OPB-31121, at the MTD of 800 mg/day, is safe and well tolerated, and has a preliminary antitumor activity. Further characterization of OPB-31121 and clinical development combined with a biomarker is warranted.
References
1. Yu H, Jove R. The STATs of cancer: new molecular targets come of age. Nat Rev Cancer. 2004; 4:97–105.
2. Mankan AK, Greten FR. Inhibiting signal transducer and activator of transcription 3: rationality and rationale design of inhibitors. Expert Opin Investig Drugs. 2011; 20:1263–75.

3. Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol. 2012; 30:1005–14.

4. Calvisi DF, Ladu S, Gorden A, Farina M, Conner EA, Lee JS, et al. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006; 130:1117–28.

6. Levy DE, Inghirami G. STAT3: a multifaceted oncogene. Proc Natl Acad Sci U S A. 2006; 103:10151–2.

7. Yu H, Pardol D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009; 9:798–809.

8. Alvarez JV, Greulich H, Sellers WR, Meyerson M, Frank DA. Signal transducer and activator of transcription 3 is required for the oncogenic effects of non-small-cell lung cancer-associated mutations of the epidermal growth factor receptor. Cancer Res. 2006; 66:3162–8.

9. Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, et al. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci U S A. 2000; 97:4227–32.

10. Mizutani Y, Bonavida B, Koishihara Y, Akamatsu K, Ohsugi Y, Yoshida O. Sensitization of human renal cell carcinoma cells to cis-diamminedichloroplatinum(II) by anti-interleukin 6 monoclonal antibody or anti-interleukin 6 receptor monoclonal antibody. Cancer Res. 1995; 55:590–6.
11. Alas S, Bonavida B. Inhibition of constitutive STAT3 activity sensitizes resistant non-Hodgkin's lymphoma and multiple myeloma to chemotherapeutic drug-mediated apoptosis. Clin Cancer Res. 2003; 9:316–26.
12. Li WC, Ye SL, Sun RX, Liu YK, Tang ZY, Kim Y, et al. Inhibition of growth and metastasis of human hepatocellular carcinoma by antisense oligonucleotide targeting signal transducer and activator of transcription 3. Clin Cancer Res. 2006; 12:7140–8.

13. Verstovsek S. Therapeutic potential of JAK2 inhibitors. Hematology Am Soc Hematol Educ Program. 2009. 636–42.

14. Kim MJ, Nam HJ, Kim HP, Han SW, Im SA, Kim TY, et al. OPB-31121, a novel small molecular inhibitor, disrupts the JAK2/STAT3 pathway and exhibits an antitumor activity in gastric cancer cells. Cancer Lett. 2013; 335:145–52.

15. Bendell JC, Hong DS, Burris HA 3rd, Naing A, Jones SF, Falchook G, et al. Phase 1, open-label, dose-escalation, and pharmacokinetic study of STAT3 inhibitor OPB-31121 in subjects with advanced solid tumors. Cancer Chemother Pharmacol. 2014; 74:125–30.

16. Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005; 7:387–97.

17. Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005; 365:1054–61.

18. Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005; 352:1779–90.
19. James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005; 434:1144–8.

20. Lin L, Liu A, Peng Z, Lin HJ, Li PK, Li C, et al. STAT3 is necessary for proliferation and survival in colon cancer-initiating cells. Cancer Res. 2011; 71:7226–37.

21. Lin L, Liu A, Peng Z, Lin HJ, Li PK, Li C, et al. STAT3 is necessary for proliferation and survival in colon cancer-initiating cells. Cancer Res. 2011; 71:7226–37.

22. Morikawa T, Baba Y, Yamauchi M, Kuchiba A, Nosho K, Shima K, et al. STAT3 expression, molecular features, inflammation patterns, and prognosis in a database of 724 colorectal cancers. Clin Cancer Res. 2011; 17:1452–62.

23. Chen J, Wang J, Lin L, He L, Wu Y, Zhang L, et al. Inhibition of STAT3 signaling pathway by nitidine chloride suppressed the angiogenesis and growth of human gastric cancer. Mol Cancer Ther. 2012; 11:277–87.

Fig. 1.
Plasma concentration versus time profiles of OPB-31121 following oral administration of OPB-31121 on day 1 (A) and on day 28 (B).
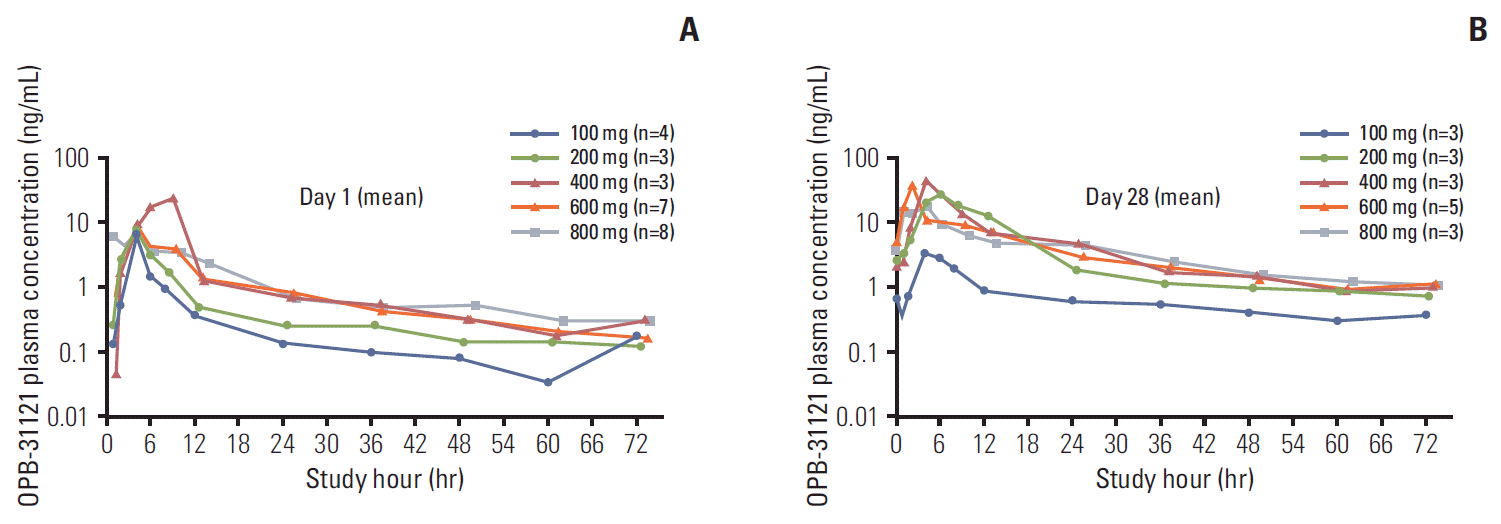
Table 1.
Patient characteristics
Table 2.
Description of patients with consent withdrawal
Table 3.
Adverse events
Table 4.
Summary of OPB-31121 plasma pharmacokinetic parameters on day 1 and day 28 and accumulation ratio in cycle 1
| OPB-31121 |
Day 1 |
Day 28 |
Accumulation ratioa) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cmax (ng/mL) | AUC∞ (ng·hr/mL) | tmax (hr) | t1/2,z (hr) | CL/F (L/hr) | Cmax (ng/mL) | AUC24hr (ng·hr/mL) | tmax (hr) | t1/2,z (hr) | CL/F (L/hr) | AUC24hr | Cmax | C24hr | |
| 100 mg | |||||||||||||
| No. | 3 | 2 | 3 | 2 | 2 | 3 | 2 | 3 | 2 | 2 | 2 | 3 | 2 |
| Meanb) | 5.9498 | 37.05 | 4.0 | 18.4 | 3,675 | 3.7617 | 35.30 | 4.0 | 40.2 | 3,435 | 1.220 | 1.565 | 3.69 |
| Min | 0.9363 | 18.0 | 2.0 | 8.77 | 1,780 | 2.121 | 20.5 | 4.0 | 39.4 | 1,990 | 1.15 | 0.374 | 3.19 |
| Max | 15.22 | 56.1 | 8.0 | 28.1 | 5,570 | 5.698 | 50.1 | 6.0 | 41.1 | 4,880 | 1.29 | 2.27 | 4.19 |
| 200 mg | |||||||||||||
| No. | 3 | 1 | 3 | 2 | 1 | 3 | 1 | 3 | 2 | 1 | 0 | 3 | 3 |
| Meanb) | 8.3047 | 28.30 | 4.10 | 32.6 | 7,060 | 25.107 | 537.0 | 4.0 | 24.8 | 372 | - | 2.323 | 5.94 |
| Min | 2.809 | - | 4.0 | 25.4 | - | 5.113 | - | 2.1 | 21.5 | - | - | 1.45 | 4.88 |
| Max | 18.57 | - | 6.0 | 39.8 | - | 64.45 | - | 6.0 | 28.2 | - | - | 3.47 | 7.68 |
| 400 mg | |||||||||||||
| No. | 3 | 1 | 3 | 1 | 1 | 3 | 1 | 3 | 2 | 1 | 1 | 3 | 2 |
| Meanb) | 32.836 | 37.90 | 6.0 | 44.3 | 1,060 | 38.890 | 242.0 | 4.0 | 66.6 | 1,660 | 1.950 | 1.643 | 4.10 |
| Min | 4.299 | - | 2.0 | - | - | 11.67 | - | 4.0 | 15.3 | - | - | 1.09 | 2.99 |
| Max | 58.13 | - | 8.0 | - | - | 65.77 | - | 4.0 | 118 | - | - | 2.71 | 5.22 |
| 600 mg | |||||||||||||
| No. | 7 | 5 | 7 | 5 | 5 | 4 | 2 | 4 | 3 | 2 | 1 | 4 | 4 |
| Meanb) | 11.671 | 76.06 | 4.0 | 17.9 | 2,489 | 36.120 | 216.5 | 2.0 | 52.6 | 1,378 | 20.20 | 6.737 | 11.46 |
| Min | 2.450 | 7.88 | 2.0 | 2.69 | 2,820 | 1.668 | 23.0 | 0.0 | 19.4 | 1,460 | - | 0.058 | 0.84 |
| Max | 28.71 | 212 | 8.0 | 26.6 | 7,610 | 109.4 | 410 | 6.0 | 88.9 | 2,610 | - | 21.4 | 28.6 |
| 800 mg | |||||||||||||
| No. | 8 | 2 | 8 | 4 | 2 | 3 | 2 | 3 | 2 | 2 | 1 | 3 | 1 |
| Meanb) | 9.4083 | 226.2 | 2.05 | 25.9 | 5,315 | 16.314 | 186.5 | 4.0 | 28.7 | 5,290 | 43.30 | 4.770 | 15.9 |
| Min | 1.534 | 95.3 | 1.0 | 15.7 | 2,240 | 6.712 | 105 | 2.0 | 15.0 | 2,980 | - | 0.521 | - |
| Max | 30.02 | 357 | 8.0 | 42.7 | 8,390 | 32.23 | 268 | 4.0 | 42.5 | 7,600 | - | 7.27 | - |
Cmax, peak (maximum) plasma drug concentration; AUC∞, area under concentration-time curve from time zero to infinity; tmax, time to peak (maximum) plasma concentration, t1/2, z, terminal-phase elimination half-life; CL/F, apparent clearance of drug from plasma after extravascular administration; AUC24hr, area under concentration-time curve from time zero to 24 hours; C24hr, concentration of drug in plasma at 24 hours; -, not determined.
Table 5.
Therapeutic efficacy of OPB31121 in patients with refractory solid tumor




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download