Abstract
Purpose
Platinum-based doublet chemotherapy is the treatment of choice for patients with non-small cell lung cancer (NSCLC); however, the role of a platinum-based doublet as second-line therapy after failure of an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) for NSCLC patients has not yet been elucidated. The purpose of this study was to compare the clinical efficacy of pemetrexed versus a platinum-based doublet as second-line therapy after failure of EGFR TKI used as first-line therapy for NSCLC patients with EGFR mutations.
Materials and Methods
We designed a multicenter retrospective cohort study of 314 NSCLC patients with EGFR mutations who received an EGFR TKI as first-line palliative chemotherapy. Our analysis included 83 patients who failed EGFR TKI therapy and received second-line cytotoxic chemotherapy.
Results
Forty-six patients were treated using a platinum-based doublet and 37 patients were treated using singlet pemetrexed. The overall response rates of patients receiving a platinum-based doublet and patients receiving pemetrexed were17.4% and 32.4%, respectively (p=0.111). The median progression-free survival (PFS) of patients receiving pemetrexed was significantly longer than that of patients receiving a platinum-based doublet (4.2 months vs. 2.7 months, respectively; p=0.008). The hazard ratio was 0.54 (95% confidence interval, 0.34 to 0.86; p=0.009).
Conclusion
Our retrospective analysis found that second-line pemetrexed singlet therapy provided significantly prolonged PFS compared to second-line platinum-based doublet chemotherapy for NSCLC patients with EGFR mutations who failed first-line EGFR TKI. Conduct of prospective studies for confirmation of our results is warranted.
Lung cancer is the second most commonly diagnosed cancer and is the leading cause of cancer-related death in both men and women [1]. Non-small cell lung cancer (NSCLC) accounts for 80% of all lung cancers, and only 30% of patients are diagnosed during the early stages of disease [2]. In 1995, platinum-based doublet cytotoxic chemotherapy was found to produce a survival benefit, and it is still being used as first-line cytotoxic chemotherapy for patients with NSCLC [3,4]. However, subsequent to the discovery of activating epidermal growth factor receptor (EGFR) mutations, recent studies have confirmed that EGFR tyrosine kinase inhibitors (TKIs) used as first-line treatment for NSCLC patients with activating EGFR mutations provided a significantly superior response rate (RR) and progression-free survival (PFS), as well as better quality-of-life scores [5-9]. Therefore, EGFR TKIs have become the preferred first-line treatment for NSCLC patients with EGFR mutations.
Among patients with advanced NSCLC, 10% of Caucasian patients and approximately 50% of Asian patients have EGFR mutations [10,11]. Although EGFR TKIs have improved outcomes for patients with EGFR mutations [7,9], few studies on optimal second-line treatments, including second-line cytotoxic chemotherapy, after failure of first-line EGFR TKI have been reported. In cases where administration of cytotoxic chemotherapy after TKI failure is being planned, platinum-based doublet chemotherapy should be considered as the first-line cytotoxic treatment. However, since cytotoxic chemotherapy is being used as a second-line treatment after EGFR TKI failure, a singlet agent such as docetaxel or pemetrexed can be used. Although there is no strong supporting evidence, current guidelines recommend use of platinum-based doublet chemotherapy after failure of first-line EGFR TKI [12]. To date, no randomized prospective studies have been reported, and the use of platinum-based doublet or singlet cytotoxic chemotherapy remains controversial.
The purpose of this study was to compare the clinical efficacy of singlet pemetrexed with the efficacy of platinumbased doublets used as second-line therapy after failure of EGFR TKI used as first-line therapy for NSCLC patients with EGFR mutations.
We performed a retrospective screening of 314 patients with advanced NSCLC and EGFR mutations, who were seen at Seoul National University Hospital (SNUH), Seoul National University Bundang Hospital (SNUBH), and Seoul National University Boramae Medical Center (SNU-BMC) from January 2006 to April 2014. The inclusion criteria were as follows: (1) activating EGFR mutations consisting of microdeletion in exon 19 or an L858R point mutation in exon 21, (2) all of the study patients had received first-line therapy using palliative EGFR TKI (gefitinib or erlotinib), and (3) all patients had failed first-line EGFR TKI treatment. A total of 83 patients were enrolled in the study. This study was approved by the Institutional Review Boards (IRBs) of SNUH, SNUBH, and SNU-BMC (SNUH IRB No. 1404-080-564; SNUBH IRB No. B-1404/246-405; SNU-BMC IRB No. 16-2014-43). The Declaration of Helsinki recommendations for biomedical research involving human subjects were also followed.
The patients’ medical records were used to collect information on the following: medical history of cancer, histopathological profile of the tumor, treatment history, and imaging studies. The EGFR gene mutations were determined using a direct sequencing method [13,14]. Patients underwent baseline computed tomography at the beginning of second-line cytotoxic chemotherapy, routine chest radiography every 3-4 weeks, and computed tomography every 2-3 cycles of chemotherapy. Evaluation of treatment response was based on the Response Evaluation Criteria in Solid Tumors (RECIST) [15]. Patients achieving complete response and partial response were considered to be responders. The primary endpoint was PFS after second-line chemotherapy. Secondary endpoints were the RR after second-line chemotherapy and overall survival (OS).
The baseline characteristics of the study population were analyzed using descriptive statistics. PFS of second-line chemotherapy was calculated from the date of initiation of second-line chemotherapy to the date of cancer progression or any cause of death. PFS was also calculated from the date of initiation of first-line TKI. OS for second-line chemotherapy was measured from the date of initiation of second-line chemotherapy to the date of death from any cause. PFS and OS were estimated using Kaplan-Meier analysis, and the difference between the survival curves of the treatment groups was tested using the log-rank test. Univariate analysis and multivariate analysis were performed using the Coxregression proportional hazards model. Statistical analysis was performed using Stata ver. 12.1 (StataCorp, College Station, TX), and all results were considered significant with a two-tailed p < 0.05.
Among the 314 patients, 83 NSCLC patients with EGFR mutations who failed first-line TKI were evaluable. Forty-six patients were subsequently treated using a platinum-based doublet (gemcitabine/cisplatin, n=21; gemcitabine/carboplatin, n=11; taxane/carboplatin, n=14) and 37 patients were treated using singlet pemetrexed. The flow chart for selection of study patients is shown in Fig. 1. Exon 19 deletion was identified in 56 patients, and a point mutation (L858R) was detected in exon 21 in 27 patients. The median age of the study patients was 62 years (range, 43 to 85 years) and the majority were women (n=52, 62.7%). The performance status (PS) of most patients was good (Eastern Cooperative Oncology Group [ECOG] PS of 0, 1), and only 15 patients (18.1%) were classified as poor (ECOG PS 2-4). A baseline evaluation for metastatic sites found that lung to lung metastasis was most common (40.9%), followed by bone, pleura (malignant pleural effusion), brain, and lymph nodes. The median PFS for first-line TKI treatment was 8.4 months (95% confidence interval [CI], 7.0 to 10.1 months) for patients receiving a platinum-based doublet and 10.0 months (95% CI, 8.2 to 13.2 months) for those receiving pemetrexed. Patients were treated with second-line cytotoxic chemotherapy for a median of 2.5 months (95% CI, 1.8 to 2.9 months); patients receiving a platinum-based doublet were treated for a median of 1.6 months (95% CI, 1.1 to 2.5 months) and those receiving pemetrexed, a median of 4.0 months (95% CI, 2.2 to 5.0 months). Among patients receiving a platinum-based doublet, 26 patients subsequently received third-line pemetrexed therapy, and six patients who received second-line pemetrexed then received third-line platinum-based doublet therapy (data not shown). Other baseline characteristics are shown in Table 1.
Out of 83 patients, 79 patients were available for evaluation of response. Twenty patients (24.1%) were responders (complete plus partial) to second-line cytotoxic chemotherapy; eight patients (17.4%) receiving platinum-based doublet therapy and 12 patients (32.4%) receiving pemetrexed were responders. Direct comparison of the overall RRs of patients receiving platinum-based doublet versus pemetrexed singlet failed to demonstrate statistical significance (p=0.111). However, more patients receiving platinum-based doublet therapy showed disease progression (n=20, 43.5%) than patients receiving pemetrexed (n=7, 18.9%) at the time of the first evaluation. The disease control rate (DCR) of patients receiving pemetrexed (n=29, 78.4%) was significantly higher than that for patients receiving a platinum-based doublet (n=23, 50.0%; p=0.008) (Table 2).
Out of 83 patients, progression of disease was observed in 77 patients. The Kaplan-Meier curves of PFS are shown in Fig. 2. The median PFS of patients receiving platinum-based doublet therapy was 2.7 months (95% CI, 1.5 to 3.2 months) and for those receiving pemetrexed was 4.2 months (95% CI, 2.9 to 6.2; p=0.008) (Table 3). In addition, the median PFS of patients receiving platinum-based doublet therapy from the date of initiation of first-line TKI treatment was 11.7 months (95% CI, 10.2 to 14.5 months) and of those receiving pemetrexed was 16.7 months (95% CI, 13.9 to 21.8 months; p=0.033) (Fig. 2).
The platinum-based doublet versus pemetrexed therapy hazard ratio (HR) for PFS was 0.54 (95% CI, 0.34 to 0.86; p=0.009); and, when PS, age, sex, and type of EGFR TKI were considered, the adjusted HR was 0.35 (95% CI, 0.20 to 0.62; p < 0.001). In subgroup analysis to examine patients with ECOG PS 0 and 1, male patients, and female patients, the HRs were 0.50 (95% CI, 0.30 to 0.83; p=0.008), 0.55 (95% CI, 0.24 to 1.27; p=0.162), and 0.41 (95% CI, 0.22 to 0.76; p=0.005), respectively (Table 4).
Out of 83 patients, there were 44 death events. The median OS was 15.1 months in the pemetrexed treated arm (95% CI, 9.7 to 26.0 months) and 11.0 months in the platinum-based doublet treated arm (95% CI, 9.4 to 21.2 months). However, the power of this study was insufficient for establishing statistical significance (p=0.785) (Table 3, Fig. 2B).
This study found that second-line singlet pemetrexed for NSCLC patients with EGFR mutations who failed first-line EGFR TKI treatment showed longer PFS compared with patients receiving a platinum-based doublet.
It is important to note that, although doublet therapy is widely used as first-line cytotoxic chemotherapy for patients with NSCLC, some reports have suggested that singlet therapy is as effective as combination therapy [16]. Because of the greater toxicity of doublet therapy, singlet chemotherapy is particularly recommended for patients with poor PS and advanced age [17-19]. Current guidelines recommend use of platinum-based doublet therapy for NSCLC patients with EGFR mutations who fail first-line TKI therapy, however, no randomized studies to provide data to support the recommendations have been reported [12]. Our retrospective study evaluated singlet pemetrexed, a third-generation cytotoxic agent with good efficacy for tumors with nonsquamous histology [20]. Our results showed that the PFS of patients receiving pemetrexed was significantly longer than that of patients receiving a platinum-based doublet. In addition, subpopulation analysis showed that the HR decreased for patients with good ECOG PS (0, 1) and for female patients.
Regarding our results, there are important points that should be considered. First, the efficacy of pemetrexed singlet treatment was greater in our study population. The DCR of patients receiving pemetrexed was significantly higher than that of those receiving a platinum-based doublet (50.0% vs. 78.4%, respectively; p= 0.008). Our results suggest that NSCLC patients with EGFR mutations have altered response to conventional cytotoxic chemotherapy, which was also seen in previous investigations [21,22]. Second, gender could be a key factor altering the response to pemetrexed and platinum. Although the larger female population enrolled in our study can be explained by a higher expression rate of EGFR mutation in Asian, non-smoker, and female patients, there is no clear explanation for improved HR with pemetrexed treatment compared with platinum doublet treatment in the female population. Conduct of further study is needed in order to prove the mechanism of different RR between sexes.
There was no statistically significant difference in the OS of our patients treated using singlet pemetrexed versus a platinum-based doublet. In addition to the small number of death events, 26 patients out of the 46 receiving a platinum-based doublet therapy were subsequently changed to third-line pemetrexed after failure of platinum doublet chemotherapy. The change to pemetrexed should be considered in interpretation of OS.
There are several study limitations. First, our study was a retrospective study and there was a limitation in balancing the baseline demographics. Although age and sex in the study population differed between the two groups, clinical characteristics of other patients were balanced between the two treatment arms. Second, despite the poor performance, 10 patients in ECOG PS 2-4 were treated with platinum doublet. Out of 10 patients, six patients were treated with a combination of taxol and platinum and four patients with gemcitabine and platinum, which was explained by different preference for selection of chemotherapy between oncologists. Third, after failure of first-line EGFR TKI, molecular profiling, which might have provided information on sensitivity to second-line cytotoxic chemotherapy, was not performed because of the difficulty of obtaining adequate samples from a second biopsy after progression. Previous studies have found that increased thymidylate synthase expression showed correlation with poor outcome and poor response to thymidylate synthase inhibitors, including pemetrexed [23,24]. Our study did not investigate the relationship between resistance to EGFR TKI and thymidylate synthase expression. Therefore, we believe that prospective studies are needed in order to confirm the hypothetical relationship between the pure effect of a thymidylate synthase inhibitor and the mechanism of resistance to EGFR TKI in patients with NSCLC. Despite the above mentioned limitations, our study is the first study to compare the effect of pemetrexed and platinum doublet in first line EGFR TKI failure patients.
In conclusion, the PFS of NSCLC patients with EGFR mutations who failed first-line EGFR TKI and then received second-line singlet pemetrexed was longer than that for similar patients who received second-line platinum-based doublet therapy. Pemetrexed singlet chemotherapy can be the second-line therapy of choice after failure of first-line EGFR TKI. Conduct of prospective studies for confirmation is warranted.
ACKNOWLEDGMENTS
This work was supported by the Innovative Research Institute for Cell Therapy, Republic of Korea (A062260); and the National Research Foundation of Korea (NRF), which is funded by the Korean government (2010-0009563).
References
2. Novello S, Le Chevalier T. Chemotherapy for non-small-cell lung cancer. Part 1: early-stage disease. Oncology (Williston Park). 2003; 17:357–64.
3. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002; 346:92–8.

4. Azzoli CG, Baker S Jr, Temin S, Pao W, Aliff T, Brahmer J, et al. American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-smallcell lung cancer. J Clin Oncol. 2009; 27:6251–66.

5. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010; 362:2380–8.

6. Han JY, Park K, Kim SW, Lee DH, Kim HY, Kim HT, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012; 30:1122–8.

7. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010; 11:121–8.

8. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012; 13:239–46.
9. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011; 12:735–42.

10. Hirsch FR, Bunn PA Jr. EGFR testing in lung cancer is ready for prime time. Lancet Oncol. 2009; 10:432–3.

11. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004; 350:2129–39.

12. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: non-small cell lung cancer. ver. 4.2014. Fort Washington, PA: National Comprehensive Cancer Network;2014.
13. Keam B, Kim DW, Park JH, Lee JO, Kim TM, Lee SH, et al. Rare and complex mutations of epidermal growth factor receptor, and efficacy of tyrosine kinase inhibitor in patients with non-small cell lung cancer. Int J Clin Oncol. 2014; 19:594–600.

14. Kim YT, Kim TY, Lee DS, Park SJ, Park JY, Seo SJ, et al. Molecular changes of epidermal growth factor receptor (EGFR) and KRAS and their impact on the clinical outcomes in surgically resected adenocarcinoma of the lung. Lung Cancer. 2008; 59:111–8.

15. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.

16. Gridelli C, Perrone F, Gallo C, Cigolari S, Rossi A, Piantedosi F, et al. Chemotherapy for elderly patients with advanced non-small-cell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Natl Cancer Inst. 2003; 95:362–72.

17. Lilenbaum R, Zukin M, Pereira JR, Barrios CH, De Albuquerque Ribeiro R, de Mendonça Beato CA, et al. A randomized phase III trial of single-agent pemetrexed (P) versus carboplatin and pemetrexed (CP) in patients with advanced non-small cell lung cancer (NSCLC) and performance status (PS) of 2. J Clin Oncol. 2012; 30 Suppl:7506.

18. Roth BJ, Krilov L, Adams S, Aghajanian CA, Bach P, Braiteh F, et al. Clinical cancer advances 2012: annual report on progress against cancer from the american society of clinical oncology. J Clin Oncol. 2013; 31:131–61.

19. Zukin M, Barrios CH, Pereira JR, Ribeiro Rde A, Beato CA, do Nascimento YN, et al. Randomized phase III trial of singleagent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol. 2013; 31:2849–53.

20. Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapynaive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008; 26:3543–51.

21. Douillard JY, Shepherd FA, Hirsh V, Mok T, Socinski MA, Gervais R, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trial. J Clin Oncol. 2010; 28:744–52.

22. Park JH, Lee SH, Keam B, Kim TM, Kim DW, Yang SC, et al. EGFR mutations as a predictive marker of cytotoxic chemotherapy. Lung Cancer. 2012; 77:433–7.

Fig. 1.
Flow chart for selection of study patients. EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; TKI, tyrosine kinase inhibitor.
Fig. 2.
FKaplan-Meier curves for progression-free survival (PFS) from the start of second-line chemotherapy (A), for overall survival (OS) from the start of second-line chemotherapy (B), for PFS from the start of first-line tyrosine kinase inhibitor (C), and for OS from the start of first-line tyrosine kinase inhibitor (D).
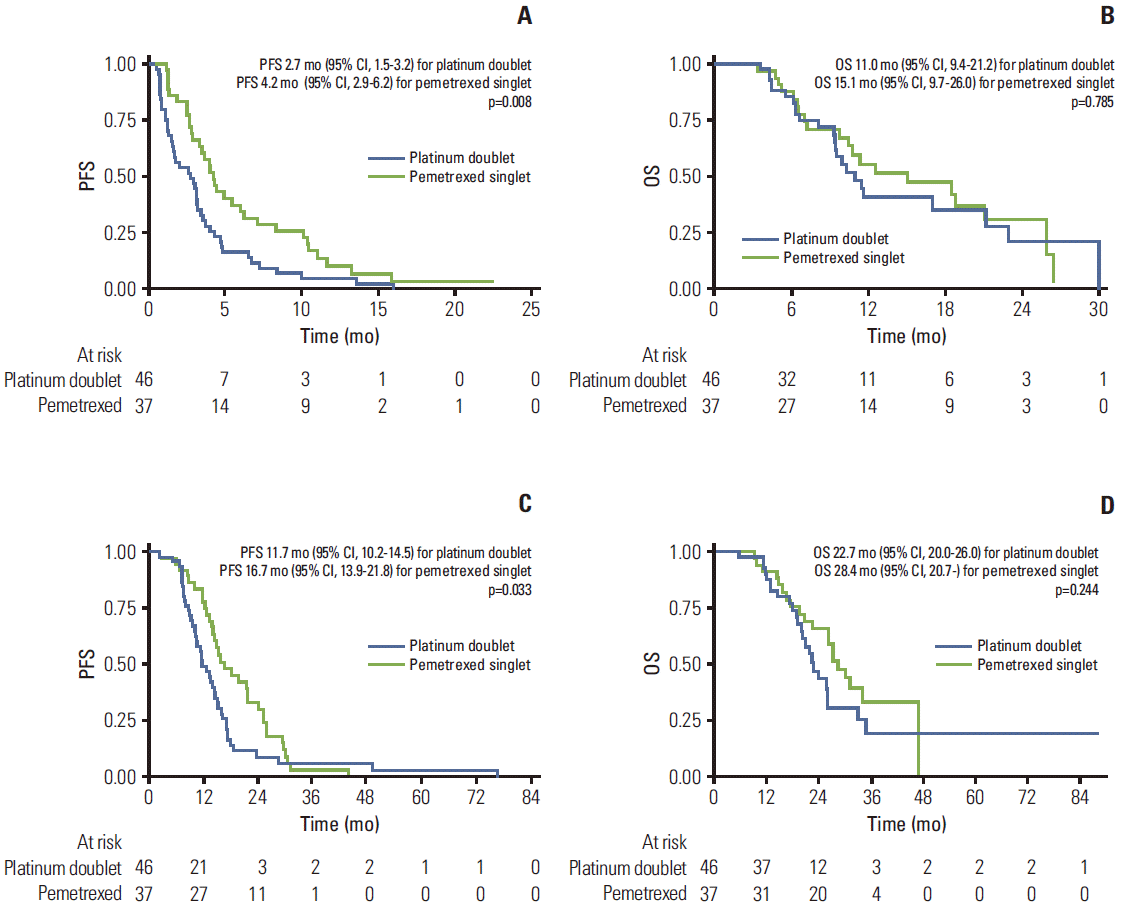
Table 1.
Baseline characteristics of patients treated with second-line cytotoxic chemotherapy after failure of first-line EGFR tyrosine kinase inhibitor
Values are presented as number (%) or median (95% confidence interval). EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; NOS, not otherwise specified; ECOG PS, Eastern Cooperative Oncology Group performance status; MPE, malignant pleural effusion; TKI, tyrosine kinase inhibitor; PFS, progression-free survival.
Table 2.
Objective tumor response of platinum doublet-treated and pemetrexed-treated patients
Table 3.
Progression-free survival, overall survival, and hazard ratio between platinum doublet-treated and pemetrexedtreated patients from the start of second-line treatment
Table 4.
Progression-free survival and hazard ratio between platinum doublet-treated and pemetrexed-treated patients in subgroups: ECOG PS 1 and 2, male and female patients




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download