Abstract
Purpose
The purpose of this study was to investigate and compare cancer treatment near the end-of-life (EOL) over a 10-year period.
Materials and Methods
Patients with advanced solid cancer at Seoul National University Hospital who received palliative chemotherapy and had died were enrolled. We categorized the consecutive patients according to two time periods: 2002 (n=57) and 2012 (n=206). Aggressiveness of cancer treatment near the EOL was evaluated.
Results
The median patient age was 62, and 65.4% of patients (n=172) were male. Time from the last chemotherapy to death (TCD) was found to have been significantly shortened, from 66.0 days to 34.0 days during 10 years (p < 0.001); 17% of patients received molecular targeted agents as the last chemotherapy regimen in 2012. The proportion of patients who received intensive care unit care within the last month increased from 1.8% in 2002 to 19.9% in 2012 (p < 0.001), and emergency room visits within the last month also increased from 22.8% to 74.8% (p < 0.001). Although hospice referral increased from 9.1% to 37.4% (p < 0.001), timing of referral was delayed from median 53 days to 8 days before death (p=0.004). Use of targeted agents as the last chemotherapy for over-two-regimen users was associated with shortened TCD (hazard ratio, 2.564; p=0.002).
According to the 2011 World Health Organization fact sheets, cancer has become a leading cause of death worldwide [1]. In Korea, cancer has been the most common cause of death since 1990, accounting for 27.6% of all deaths in 2012 [2].
Since the Vatican’s 1980 Declaration reaffirmed that disproportionate means of preserving life, which are understood as offering no reasonable hope of benefit or involving excessive burdens on the family or community, are not obligated to a dying person, this tradition became a moral framework of end-of-life (EOL) care [3]. However, despite knowing that aggressive treatment near the EOL could not help patients survive, a majority of studies on EOL care have reported that terminal stage cancer patients still receive aggressive treatments that may do them more harm than good [4-8]. Some studies have also reported that patients dying with cancer have been administered systemic chemotherapy even up to the last 2 weeks of life [7,8]. As a result, chemotherapy without careful consideration of the entire clinical course may give patients the false hope of prolonging life, delay hospice referral, and deprive patients of opportunities to prepare for their own deaths [9,10].
Near the EOL, timely cessation of chemotherapy and hospice referral allows patients and their families to receive sufficient physical support and prepare for death emotionally and spiritually [4,11]. Recently, cancer treatment near the EOL seems to have changed with development of monoclonal antibodies and small molecules for various molecular targets. However, there are no available data regarding cancer treatment near the EOL. We conducted this study to investigate and compare changes in cancer treatment near the EOL during 10 years and their effects on EOL care at a single institute.
We assessed patients from two separate periods, i.e., 2002 and 2012. We evaluated all advanced cancer patients who died from January 1 to December 31, 2012. We also assessed patients who were diagnosed from January 1 to December 31, 2002, and followed up until death. The latter cohort had been previously assessed for investigation of EOL care [12]. The same criteria of inclusion and exclusion were applied to both groups as follows. We enrolled only patients who had been treated and died in Seoul National University Hospital. We excluded patients with (1) hematologic malignancy (leukemia, lymphoma, multiple myeloma, etc.), (2) no pathologic reports, (3) only supportive care without chemotherapy, and (4) no cancer-related death.
We reviewed medical records of these two groups in order to obtain data on EOL care indicators of aggressiveness in terms of the following aspects: intensive care unit (ICU) admission in the last month of life, emergency room (ER) visits in the last month of life, numbers of regimens and cycles, duration of chemotherapy, time from the last chemotherapy to death (TCD), hospice referral, period between hospice contact and death, and discussion of advance directives. Duration of chemotherapy was calculated from the starting day of first-line chemotherapy regimen until the last administration day of chemotherapeutic drug. TCD was counted from the last administration day of chemotherapeutic drug to death.
We examined between-group associations of demographic and clinical variables using Fisher exact tests for categorical variables and independent t test for continuous variables. All of our participants have exact dates of death with none missing. We calculated median overall survival (OS) and TCD using the Kaplan-Meier method. Log-rank test was used for comparisons of survival between groups. Hazard ratio (HR) and 95% confidence interval (CI) was calculated using the Cox proportional hazards model to examine the effect of multiple factors on survival. All tests were 2-sided, and p ≤ 0.05 was considered statistically significant. We performed statistical analyses using SPSS ver. 19.0 (IBM Corp., Armonk, NY).
The study was reviewed and approved by the Institutional Review Board of Seoul National University Hospital (IRB No. H-1310-068-527). All studies were conducted according to the guidelines of the Declaration of Helsinki for biomedical research.
A total of 263 patients were enrolled in this study. Among 358 advanced cancer patients who died in admission status in 2012, 206 patients were enrolled in the 2012 group. Out of the previous cohort of 298 patients diagnosed in 2002, 57 patients who died in our hospital were included in the 2002 group.
The median age was 63 years (range, 17 to 88 years) at diagnosis and 64 years (range, 18 to 89 years) at death. Male was 65.4% (n=172). Their primary tumors consisted of 91 (34.6%) lung cancer, 18 (6.8%) breast cancer, 27 (10.3%) colorectal cancer, 84 (31.9%) non-colorectal gastrointestinal (GI) cancer, and 43 (16.4%) other cancers (Table 1).
Age and sex distribution did not differ between the two groups with a 10-year gap (Table 2). Median ages at diagnosis and death also did not differ significantly between the groups, with 63 years (range, 23 to 76 years) versus 63 years (range, 17 to 88 years) at diagnosis (p=0.288) and 63 years (range, 23 to 76 years) versus 65 years (range, 18 to 63 years) at death (p=0.256), respectively (Table 3). Lung cancer still accounted for the main portion of hospital deaths in both groups within the 10-year interval. While breast cancers and colon cancers increased, non-colon GI cancers decreased without statistical significance (Table 2).
Mean numbers of regimens and cycles increased significantly, from 1.8 regimens (range, 1 to 6) to 2.5 regimens (range, 1 to 9) (p=0.003) and from 5.5 cycles (range, 1 to 16) to 11.6 cycles (range, 1 to 69) (p < 0.001). Median duration of chemotherapy of advanced cancer patients also showed a significant increase, from 5.5 months (range, 0.3 to 18.1 months) to 6.8 months (range, 0.03 to 84.9 months) (p=0.006). Median OS increased from 9.1 months (95% CI, 5.5 to 12.8 months) to 10.3 months (95% CI, 8.8 to 11.8 months) (p < 0.001) (Table 3).
Median TCD has become significantly shortened, from 66 days (95% CI, 49 to 83 days) to 34 days (95% CI, 30 to 39 days) (p=0.004) (Table 3). Among the sub-intervals of TCD, deaths within 2 weeks of receiving chemotherapy showed a significant increase, from 3.5% to 23.8%, while deaths more than 8 weeks from the last chemotherapy decreased from 56.1% to 28.6% (p < 0.001) (Table 2). Median TCD ratio (time from last chemotherapy to death/overall survival) decreased to less than half the initial value, from 0.325 to 0.116 (p < 0.001) (Table 3). In particular, 35 patients (17.0%) received molecular targeted agents as the last chemotherapy regimen in 2012, while none of the patients received molecular targeted agents in 2002 (Table 2). Over half of targeted agents in the last month were used for lung cancer (20 patients, 57.1%), 11 gefitinib cases, five erlotinib cases, and four crizotinib cases in lung cancer and four sorafenib, three sunitinib, one pazopanib, two everolimus, one MDM2 inhibitor, two cetuximab, and one trastuzumab in non-lung cancer (Table 4).
Median duration of the last admission increased as double, from six days to 12 days (p=0.041) (Table 3). More than one ER visit during the last months of life also increased more than three-fold, from 22.8% to 74.8% (p < 0.001). The proportion of patients who received ICU care within the last month increased more than 11 times, from 1.8% to 19.9% (p=0.001). ICU care was more frequent for patients who had visited the ER (21.0% vs. 7.3%; p=0.003) but less frequent for patients referred to hospice (8.3% vs. 79.6%; p=0.020). Median duration of ICU admission of 2012 was 5 days (range, 1 to 34 days; mean, 9; SD, 9) (Table 2). Although hospice referrals increased from 12.3% to 37.4% (p < 0.001), timing of hospice contact was delayed from median 60 days to 8 days before death (p=0.004). In 2012, 41.6% of hospice contacts were made within 1 week before death, while only 16.9% of hospice contacts were made more than 4 weeks from death. (Tables 2 and 3). Of note, 95.1% of patients documented advance directives in 2012, but no patients wrote them in 2002 (Table 2).
We performed multivariate analyses for patients in the 2012 group to determine the factors influencing the TCD. Two-or-less-regimen users had shorter TCD (HR, 0.704; 95% CI, 0.525 to 0.945; p=0.020). Use of targeted agents as the last chemotherapy and ICU care within the last month showed a tendency to shorter TCD (19 days vs. 35 days, p=0.086 and 23 days vs. 36 days, p=0.097, respectively) (Table 5, Fig. 1A-C).
Further analyses with subgroups showed that using targeted agents as the last chemotherapy for the over-tworegimen users was significantly associated with shorter TCD (HR, 2.564; 95% CI, 1.415 to 4.645; p=0.002) and ICU care within the last month had similar association with TCD in two-or-less-regimen users (HR, 1.586; 95% CI, 1.043 to 2.358; p=0.031) (Table 6, Fig. 1D).
In this study, we found that cancer care near the EOL became more aggressive compared to 10 years ago with respect to chemotherapy, ICU admissions, ER visits, and the timing of hospice referral. TCD became shorter, and use of targeted agents was the factor associated with reducing TCD for heavily treated patients. As a result, use of ICU and ER increased and hospice referral was delayed.
We found that 23.8% of the study population received chemotherapy in the last 2 weeks of life, 42.7% in the last month, and 71.4% in the last 2 months, which is higher than the rates reported by a previous study from our institute [12] and a multi-institutional Korean study of 2004 [7] and is also higher than the rates reported by studies worldwide [4,5,8].
A remarkable finding of our study is that use of targeted agents as the last chemotherapy was associated with shortened TCD in multivariate analysis. In particular, the choice of targeted agents as third or more regimen showed a significant association with reduced TCD days vs. 35 days (p=0.002). Other recent studies have also reported that targeted agents are used in the last month of life up to twice as much as non-targeted agents and are used even in the palliative care unit because of their tolerable toxicities [13,14]. NSCLC patients who are the major consumers of targeted agents deplete their healthcare resources on anti-cancer treatment during the terminal stage of disease [15].
Aggressive EOL care including prolonged duration of chemotherapy places a substantial economic burden on patients and their health insurance authority. The majority of the EOL healthcare expenditure was associated with acute care including ER and ICU care and chemotherapies [16,17]. The cost saving effect of EOL discussions and use of palliative care unit were reported as totaling more than 30% [18,19]. There is increasing evidence that early palliative care optimized the timing of chemotherapy cessation and transition to hospice services leading to longer TCD with better quality of life [20].
We should pose the big question: what drives this aggressive trend? Decreased financial burden may be a possible explanation for the increasing use of the acute care unit and chemotherapy including targeted agents near the EOL. The Korean government gradually reinforced health insurance for cancer treatment by reducing the patient burden from 20% to 10% in 2005, and from 10% to 5% in 2009. The National Evidence-based Healthcare Collaborating Agency of Korea has released data on how the reinforcement of health insurance covering cancer treatment has influenced medical service patterns up until 2010. They showed that expenditure in ICU care near the EOL was still increasing until 2010. The cost of chemotherapy both as total cost and as per person cost during the last month also increased after the first reinforcement in 2005 and use of targeted agents has been sharply increasing since 2005. However, the total cost of chemotherapy near the EOL has shown a slight reduction for the first time after the second reinforcement in 2009 but the costs of targeted agents and non-targeted agents were not separated. Therefore, we require separate cost data for targeted agents [21]. We can presume the influence of the changes in health insurance from the previous research. Private health insurance has been proven to exert a significant effect on raising the expenditure in inpatient healthcare services [22,23]. Another prior assessment of the impact of the sharp cutbacks in chemotherapy reimbursement showed that chemotherapy in the last 2 weeks of life was reduced by 20% in patients who were treated in physicians’ offices [24]. Therefore, the reinforced insurance may have led both patients and physicians to become insensitive to the cost. The low barriers to healthcare utilization in Korea could also be found in Organization for Economic Co-operation and Development (OECD) Health Data 2013 comparing the year 2000 and 2010. OECD data showed a rapid increase of the number of hospitals, hospital beds, outpatient visits, discharges, average length of hospital stay, and health expenditure per person in Korea [25]. Fee-for-service system of Korea could also promote aggressive EOL cancer treatment.
The current study has some potential limitations. First, there is the inevitable limitation of a retrospective approach. Nevertheless we consider that the retrospective approach could reveal the true extent of damage of aggressive EOL care compared to a prospective study with selective patients. Second, our study was conducted in a single tertiary referral hospital that serves as a national central hospital. The cancer treatment near the EOL of our study population could be biased toward the aggressive side because patients who expect more aggressive treatments were referred to our hospital from across the country. Third, patients in the 2012 group are those who died in 2012 but the patients of the 2002 group are those who were diagnosed in 2002. When we added the condition of ‘death within the year of 2002’ to in-hospital death to the 2002 group, we identified similar rates of the indicators of aggressiveness to this report. However, using both conditions, our study included only patients who survived within 1 year. Therefore, we used the results of pre-analysis only as the indicators of comparison, leaving the method for selection of patients as a limitation of our study. However, the median OS of the 2012 group was less than 1 year and survival of most patients did not exceed 6 months from 1 year (1-year OS of 36.8% and 18-month OS of 5.3%). Therefore, we think that our study could still have the value of a 10-year comparison. In addition, the fact that both groups have been treated in the medical oncology department and that they include a similar portion of lung cancer patients who were major consumers of targeted agents could reduce the errors caused by the heterogeneity of participants. Fourth, of note, we have not included the exact expenditure, particularly the cost of targeted agents during the last month, which is expected to explain the relationship between enforcement of health insurance and EOL cancer treatment. Fifth, while the majority of patients had agreed to the advance directives and the increased patients by 25% were referred to hospice in our study, the aggressiveness of EOL care has increased. It implies that although awareness for hospice and palliative care has improved in our society, there is still a lack of understanding with regard to advance care planning. As timing of hospice contact was delayed from 60 days to eight days before death, patients and their families could already have spent most of their time on chemotherapy and ICU care. In consequence, discussions of EOL and the referrals to hospice were too delayed to prevent aggressive treatments.
Despite these limitations, to the best of our knowledge, this is the first study to demonstrate that the use of targeted agents for heavily-treated patients near the EOL is associated with shorter TCD.
Cancer treatment near the EOL became aggressive over 10 years. ICU care and chemotherapy near the EOL has increased during a 10-year period, while hospice referral has been delayed. Use of targeted agents for heavily treated patients should be reconsidered more carefully. Further nation-wide investigations regarding the influence of financial burdens are warranted.
ACKNOWLEDGMENTS
This study was supported by a grant from the Innovative Research Institute for Cell Therapy, Republic of Korea (A062260).
References
1. World Health Organization. The 10 leading causes of death in the world, 2000 and 2011 [Internet]. Geneva: World Health Organization;2013. [cited 2014 Jul 27]. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/.
2. Statistics Korea. Annual report on the cause of death statistics (2012). Daejeon: Statistics Korea;2013. p. 127–43.
3. Sacred Congregation for the Doctrine of the Faith. Vatican declaration on euthanasia. Origins. 1980; 10:154–7.
4. Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004; 22:315–21.

5. Nieder C, Tollali T, Dalhaug A, Haukland E, Aandahl G, Pawinski A, et al. Active anticancer treatment during the final month of life in patients with non-small cell lung cancer. Anticancer Res. 2014; 34:1015–20.
6. Emanuel EJ, Young-Xu Y, Levinsky NG, Gazelle G, Saynina O, Ash AS. Chemotherapy use among Medicare beneficiaries at the end of life. Ann Intern Med. 2003; 138:639–43.

7. Yun YH, Kwak M, Park SM, Kim S, Choi JS, Lim HY, et al. Chemotherapy use and associated factors among cancer patients near the end of life. Oncology. 2007; 72:164–71.

8. Nappa U, Lindqvist O, Rasmussen BH, Axelsson B. Palliative chemotherapy during the last month of life. Ann Oncol. 2011; 22:2375–80.
9. Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008; 300:1665–73.

10. Casarett DJ, Fishman JM, Lu HL, O'Dwyer PJ, Barg FK, Naylor MD, et al. The terrible choice: re-evaluating hospice eligibility criteria for cancer. J Clin Oncol. 2009; 27:953–9.

11. Teno JM, Clarridge BR, Casey V, Welch LC, Wetle T, Shield R, et al. Family perspectives on end-of-life care at the last place of care. JAMA. 2004; 291:88–93.

12. Keam B, Oh DY, Lee SH, Kim DW, Kim MR, Im SA, et al. Aggressiveness of cancer-care near the end-of-life in Korea. Jpn J Clin Oncol. 2008; 38:381–6.

13. Hui D, Karuturi MS, Tanco KC, Kwon JH, Kim SH, Zhang T, et al. Targeted agent use in cancer patients at the end of life. J Pain Symptom Manage. 2013; 46:1–8.

14. Soh TI, Yuen YC, Teo C, Lim SW, Chan N, Wong AS. Targeted therapy at the end of life in advanced cancer patients. J Palliat Med. 2012; 15:991–7.

15. Wong AS, Teo C, Lim SW, Wong E, Soo RA, Chan N. Targeted therapy at the end of life for patients with lung cancer. J Palliat Med. 2010; 13:945–8.

16. Walker H, Anderson M, Farahati F, Howell D, Librach SL, Husain A, et al. Resource use and costs of end-of-Life/palliative care: Ontario adult cancer patients dying during 2002 and 2003. J Palliat Care. 2011; 27:79–88.

17. Morishima T, Lee J, Otsubo T, Imanaka Y. Association of healthcare expenditures with aggressive versus palliative care for cancer patients at the end of life: a cross-sectional study using claims data in Japan. Int J Qual Health Care. 2014; 26:79.

18. Zhang B, Wright AA, Huskamp HA, Nilsson ME, Maciejewski ML, Earle CC, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med. 2009; 169:480–8.
19. Jung HM, Kim J, Heo DS, Baek SK. Health economics of a palliative care unit for terminal cancer patients: a retrospective cohort study. Support Care Cancer. 2012; 20:29–37.

20. Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010; 363:733–42.

21. National Evidence-based Healthcare Collaborating Agency. Evaluation of the effect on medical service of the enforcement of health insurance on cancer [Internet]. Seoul: National Evidence-based Healthcare Collaborating Agency;2011. [cited 2014 Jul 27]. Available from: http://hineca.kr/m/post/164#.
22. Lim JH, Choi KS, Kim SG, Park EC, Park JH. Effects of private health insurance on health care utilization and expenditures in Korean cancer patients: focused on 5 major cancers in one cancer center. J Prev Med Public Health. 2007; 40:329–35.

23. Chirikos TN. Cancer economics: on variations in the costs of treating cancer. Cancer Control. 2002; 9:59–66.

24. Colla CH, Morden NE, Skinner JS, Hoverman JR, Meara E. Impact of payment reform on chemotherapy at the end of life. Am J Manag Care. 2012; 18:e200–8.

25. Organization for Economic Co-operation and Development. OECD Health Data 2013 [Internet]. Paris: Organization for Economic Co-operation and Development;2013. [cited 2014 Jul 27]. Available from: http://stats.oecd.org/index.aspx?DataSetCode=HEALTH_STAT
.
Fig. 1.
Factors associated with time from last chemotherapy to death in 2012. (A) Time from last chemotherapy to death by number of regimens. (B) Time from last chemotherapy to death by use of targeted agents as the last chemotherapy. (C) Time from last chemotherapy to death by intensive care unit (ICU) care within the last month. (D) Time from last chemotherapy to death by use of targeted agents as the last chemotherapy (as third or more regimen).
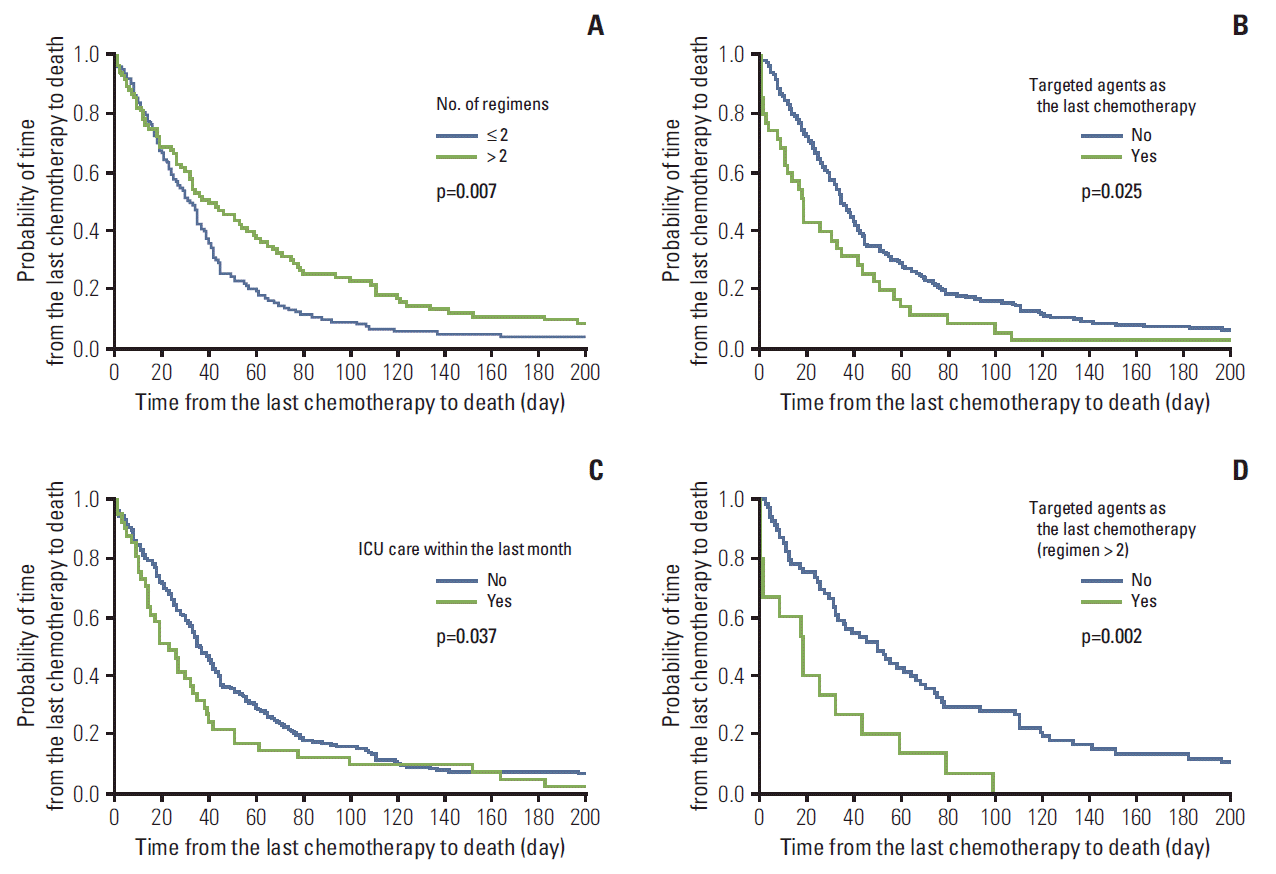
Table 1.
Baseline demographic and clinical characteristics (n=263)
| Characteristic | No. (%) |
|---|---|
| Gender | |
| Male | 172 (65.4) |
| Female | 91 (34.6) |
| Age of diagnosis (yr) | |
| < 60 | 106 (40.3) |
| ≥ 60 | 157 (59.7) |
| Age of death (yr) | |
| < 60 | 99 (37.6) |
| ≥ 60 | 164 (62.4) |
| Diagnosis | |
| Lung | 91 (34.6) |
| Breast | 18 (6.8) |
| Colon | 27 (10.3) |
| Non-colon GIa) | 84 (31.9) |
| Elseb) | 43 (16.4) |
Table 2.
Ten-year changes of categorical variables on end-of-life care (n=263)
| Variable |
2002 group (n=57) |
2012 group (n=206) |
p-valuea) |
|---|---|---|---|
| Gender | 0.273 | ||
| Male | 41 (71.9) | 131 (63.6) | |
| Female | 16 (28.1) | 75 (36.4) | |
| Age of diagnosis (yr) | 0.879 | ||
| < 60 (n=106) | 22 (38.6) | 84 (40.8) | |
| ≥ 60 (n=157) | 35 (61.4) | 122 (59.2) | |
| Age of death (yr) | > 0.999 | ||
| < 60 (n=99) | 21 (36.8) | 78 (37.9) | |
| ≥ 60 (n=164) | 36 (63.2) | 128 (62.1) | |
| Diagnosis | 0.112 | ||
| Lung | 18 (31.6) | 73 (35.4) | |
| Breast | 1 (1.8) | 17 (8.3) | |
| Colon | 3 (5.3) | 24 (11.7) | |
| Non-colon GIb) | 24 (42.1) | 60 (29.1) | |
| Othersc) | 11 (19.3) | 32 (15.5) | |
| Time from last chemotherapy to death (wk)d) | < 0.001 | ||
| ≤ 2 | 2 (3.5) | 49 (23.8) | |
| 2-4 | 6 (10.5) | 39 (18.9) | |
| 4-8 | 17 (29.8) | 59 (28.6) | |
| > 8 | 32 (56.1) | 59 (28.6) | |
| Targeted agentse) as the last chemotherapy | < 0.001 | ||
| Yes | 0 | 35 (17.0) | |
| No | 57 (100) | 171 (83.0) | |
| ER visits within the last month | < 0.001 | ||
| Done | 13 (22.8) | 154 (74.8) | |
| Not done | 44 (77.2) | 52 (25.2) | |
| ICU care within the last month | 0.001 | ||
| Done | 1 (1.8) | 41 (19.9) | |
| Not done | 56 (98.2) | 165 (80.1) | |
| Hospice referral | < 0.001 | ||
| Done | 7 (12.3) | 77 (37.4) | |
| Not done | 50 (87.7) | 129 (62.6) | |
| Period between hospice contact and death (wk) | 0.003 | ||
| ≤ 1 | 0 | 32 (41.6) | |
| 1-4 | 2 (28.6) | 32 (41.6) | |
| > 4 | 5 (71.4) | 13 (16.9) | |
| Advance directives | < 0.001 | ||
| Yes | 0 | 196 (95.1) | |
| No | 57 (100) | 10 (4.9) |
Values are presented as number (%). GI, gastrointestinal; ER, emergency room; ICU, intensive care unit.
Table 3.
Ten-year changes in median values of continuous variables on end-of-life care
| Variable | 2002 group (n=57) | 2012 group (n=206) | p-valuea) | ||||
|---|---|---|---|---|---|---|---|
| Median (range) | 95% CI | Mean±SD | Median (range) | 95% CI | Mean±SD | ||
| Age of diagnosis (yr) | 63 (23-76) | - | 59±12 | 63 (17-88) | - | 61±13 | 0.288 |
| Age of death (yr) | 63 (23-76) | - | 60±12 | 65 (18-89) | - | 63±13 | 0.256 |
| No. of regimensb) | - | - | 1.8±1.01 | - | - | 2.5±1.7 | 0.003 |
| No. of cyclesb) | - | - | 5.5±4.0 | - | - | 11.6±12.1 | < 0.001 |
| Duration of chemotherapy (mo)c) | 5.5 (0.3-18.1) | - | 6.6±4.8 | 6.8 (0.03-84.9) | - | 12.4±15.5 | 0.006 |
| Overall survival (mo)d) | 9.11 | 5.5-12.8 | 9.4±5.3 | 10.3 | 8.8-11.8 | 17.5±19.2 | < 0.001a) |
| Time from last chemotherapy to death (day)d),f) | 66 | 49-83 | 84±61 | 34 | 30-39 | 60±87 | 0.004a) |
| Time from last chemotherapy to death ratiog) | 0.325 (0.011-0.878) | - | 0.359±0.233 | 0.116 (0-0.812) | - | 0.181±0.179 | < 0.001 |
| Duration of the last admission (day) | 6 (1-59) | - | 11±14 | 12 (1-108) | - | 16±16 | 0.041 |
| Period between hospice contact and death (day)a) | 60 | 0-134 | 84±99 | 8 | 7-9 | 21±48 | 0.004a) |
Table 4.
Targeted agents used during the last month in 2012
Table 5.
Factors associated with chemotherapy-free survival
| Variable | Chemotherapy-free survival (day) | Median OS of last chemotherapy (day) |
Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-valuea) | HR (95% CI) | p-valuea) | |||||
| No. of regimens | 0.007 | 0.020 | ||||||
| > 2 | 40.0 (25.9-54.1) | 78.0 (64.7-91.3) | 0.677 (0.509-0.901) | 0.704 (0.525-0.945) | ||||
| < 2 | 32.0 (26.6-37.4) | 83.0 (62.9-103.1) | 1 | 1 | ||||
| Targeted agents as the last chemotherapy | 0.025 | 0.086 | ||||||
| Yes | 19.0 (13.3-24.7) | 77.0 (43.4-110.6) | 1.524 (1.055-2.200) | 1.387 (0.954-2.016) | ||||
| No | 35.0 (30.3-39.7) | 82.0 (68.6-95.5) | 1 | 1 | ||||
| Hospice referral | 0.174 | 0.409 | ||||||
| Yes | 42.0 (33.4-50.6) | 88.0 (68.8-107.2) | 0.820 (0.616-1.091) | 0.884 (0.661-1.184) | ||||
| No | 28.0 (22.0-34.0) | 75.0 (56.7-93.3) | 1 | 1 | ||||
| ICU care within the last month | 0.037 | 0.097 | ||||||
| Yes | 23.0 (14.0-32.0) | 64.0 (25.1-102.9) | 1.443 (1.022-2.039) | 1.345 (0.948-1.911) | ||||
| No | 36.0 (30.4-41.6) | 85.0 (70.5-99.5) | 1 | 1 | ||||
| ER visit within the last month | 0.539 | 0.404 | ||||||
| Yes | 34.0 (29.5-38.5) | 82.0 (67.2-92.8) | 0.905 (0.656-0.244) | 0.869 (0.624-1.209) | ||||
| No | 32.0 (21.4-42.6) | 78.0 (38.0-118.0) | 1 | 1 | ||||
Table 6.
Factors associated with chemotherapy-free survival
| Variable |
Regimen ≤ 2 |
Regimen > 2 |
||||
|---|---|---|---|---|---|---|
| Chemotherapy-free survival (day) | HR (95% CI) | p-valuea) | Chemotherapy-free survival (day) | HR (95% CI) | p-valuea) | |
| Targeted agents as the last chemotherapy | 0.742 | 0.002 | ||||
| Yes | 17.0 (6.0-28.0) | 1.086 (0.663-1.778) | 19.0 (6.6-31.4) | 2.564 (1.415-4.645) | ||
| No | 34.0 (29.0-40.0) | 1 | 35.0 (28.8-41.2) | 1 | ||
| ICU care within the last month | 0.031 | 0.674 | ||||
| Yes | 19.0 (7.9-30.1) | 1.586 (1.043-2.358) | 32.0 (14.5-49.5) | 0.859 (0.422-1.748) | ||
| No | 35.0 (28.8-41.2) | 1 | 40.0 (23.1-56.9) | 1 | ||




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download