Abstract
Purpose
We report the outcomes of patients treated with palliative radiotherapy (pRT) to the primary tumour in the context of well-controlled metastatic disease after initial chemotherapy.
Materials and Methods
Clinical records of 132 patients with metastatic esophago-gastric (OG) cancer treated with palliative chemotherapy (pCT) between January 2009 and June 2013 were reviewed. Ninetyseven patients had responding or stable disease after 3 months of chemotherapy, of whom 53 patients received pRT to the primary tumour after initial chemotherapy in the presence of well-controlled metastatic disease (group A, pCT-RT). The remaining 44 patients were treated with pCT alone (group B, pCT). Treatment-related outcomes were assessed in above groups including time to local progression (TTLP), progression-free and overall survival.
Results
The median overall survival for patients treated with pRT after initial chemotherapy (group A) was 23.3 months (95% confidence interval [CI], 17.70 to 28.89 months) and significantly higher than the 14 months (95% CI, 10.91 to 17.08 months) in patients treated with pCT alone (group B) (p < 0.001). The use of pCT-RT was an independent predictor of OS in multivariate analysis. Local recurrence was observed in 12/53 of patients (23%) in group A compared to 16/44 (36%) in group B. The median TTLP was significantly higher in patients after pCT-RT at 17.3 months (5.23 months to 44.50 months) compared to 8.3 months (range, 4.10 to 25.23 months) in patients treated with pCT alone (p=0.006).
The last decade has witnessed significant improvements in survival outcomes of patients with localized esophageal and gastric cancers, possibly related to the more frequent use of multimodal therapy. However, the prognosis of metastatic esophago-gastric (OG) cancer remains poor. Therapeutic strategies typically employed in this situation include systemic chemotherapy, palliative external-beam radiotherapy, high dose-rate brachytherapy (HDR-BT), endoscopic therapies including endoluminal stenting (ELS), argon plasma coagulation, photodynamic therapy, and occasionally surgery.
The recent National Comprehensive Cancer Network guidelines (NCCN) ver. 2.2013 recommend chemotherapy as a therapeutic option for palliating symptoms, improving quality of life (QoL), and prolonging survival in patients with metastatic OG cancer incorporating esophageal, gastro-esophageal junction (GOJ), and gastric tumours [1]. Published response rates of palliative chemotherapy (pCT) in metastatic OG cancer range from 40%-60% with median life expectancy ranging from 7-12 months [2-7].
The chemotherapy protocols commonly employed include the use of doublet regimes incorporating platinum agent (cisplatin or oxaliplatin) combined with fluoropyrimidine (5 fluorouracil [5 FU] or capecitabine), or triplet regimes including the use of a third drug, usually in the form of epirubicin, docetaxel, or mitomycin. The recent Trastuzumab for Gastric Cancer (ToGA) trial demonstrated a survival benefit from addition of trastuzumab to standard doublet regime of platinum/fluoropyrimidine (FP) chemotherapy in patients with HER-2 positive metastatic GOJ and gastric adenocarcinomas (AC) [3]. In contrast to pCT, palliative radiotherapy (pRT) is primarily employed for symptom control in patients presenting with local symptoms of dysphagia or bleeding.
In our centre, patients with metastatic OG cancer with good performance status (PS) are treated with pCT consistent with NCCN recommendations and local guidelines. Subsequently, pRT may be employed in those patients developing a good response to initial chemotherapy with the aim of optimising local control. We have observed significantly improved outcomes in patients treated with pRT in the presence of well-controlled metastatic disease after initial chemotherapy. We report on the outcomes of such patients and compare them against a comparable favourable cohort of patients treated with pCT alone.
Before conducting the current study, a retrospective audit review protocol was designed and approved for evaluation of outcomes of patients with metastatic OG cancer treated with pCT. Between January 2009 and June 2013, 132 patients were identified with histopathologically confirmed diagnoses of metastatic OG cancer who went on to receive pCT. The clinical evaluation of these patients included a complete medical history, physical examination, laboratory investigations, diagnostic endoscopy, and appropriate radiological imaging including computed tomography (CT) scan of the chest, abdomen and pelvis. Subsequently, all patients were discussed at the local multidisciplinary team (MDT) where the histopathology and radiological imaging were reviewed, and in a few instances further diagnostic investigations were requested (positron-emission tomography, magnetic resonance imaging, and bone scan), as appropriate depending on the discretion of the MDT and the treating physician.
Most patients commenced chemotherapy within six weeks of diagnosis and received standard platinum-based combination chemotherapy regimes for metastatic OG cancer. Patients underwent regular clinical assessments and staging CT scan at appropriate time-intervals (3-4 months) for objective assessment of response to chemotherapy. From the original 132 patients, 35 patients experienced rapid disease progression within the first 3 months characterised by rapid clinical deterioration and poor prognosis with median life-expectancy of less than 6 months. These patients were excluded from further evaluation and survival analysis. In addition, there were 97 patients with stable or responding disease at 3 months after initial chemotherapy, of whom 53 patients received pRT to primary tumour in the presence of stable and well-controlled metastatic disease (group A, pCT-RT). In contrast, the remaining 44 patients were treated primarily with pCT alone (group B, pCT) (Fig. 1A).
Most patients underwent virtual simulation for target definition and radiotherapy portals were defined to include the primary tumour (entire stomach in gastric cancer) and any adjacent areas of residual disease that could be safely encompassed in the radiotherapy field with 1.5- to 2-cm margin. The radiotherapy dose was prescribed to midplane at the isocentre and treatment was delivered using parallelopposed fields and 6-MV photons with wedge compensation employed in selected cases for ensuring homogenous dose-distribution within the irradiated volume. Patients with gastric tumours treated with higher radiotherapy dosage (30 Gy in 10 fractions [n=10], 45 Gy in 25 fractions [n=1]) underwent CT planning for target definition to reduce dose to adjacent critical organs. The planning target volume was defined to including the entire stomach and any adjacent areas of residual disease with 1.5-cm isotropic margin. The target volume was treated using 3-dimensional-conformal radiotherapy with 3- to 4-field beam arrangement and 6-MV photons (Table 1).
The evaluation included a comprehensive assessment of patient demographics, tumour-related characteristics, therapeutic modalities employed including chemotherapy (regime, duration and number of cycles of 1st and subsequent lines of chemotherapy) and radiotherapy (dose-fractionation, technologies, planning and dosimetric characteristics). Furthermore, treatment-related outcomes were analysed, including progression-free survival (PFS), overall survival (OS), and time to local progression (TTLP).
Descriptive statistics such as the mean±standard deviation (parametric), median (range) (nonparametric), and frequency (percentage) (categorical) were employed to summarize the distribution of various patient, tumour, and treatment-related variables. Further statistical analysis was performed using the chi-square test for categorical variables and the Mann-Whitney U-test for continuous nonparametric variables. For the analysis of OS, disease-specific death was the only event noted, with surviving patients censored at the time of last visit. The date of disease progression or recurrence, as demonstrated by clinical deterioration and radiological imaging, was defined as an event for the analysis of PFS. The duration of PFS and OS was estimated from date of diagnosis, and Kaplan-Meier technique was used to estimate the PFS probabilities, combined with the univariate and multivariate Cox proportional hazards models to measure the association of various clinical parameters with PFS and OS. The log-rank test was used to assess the difference in PFS and OS among different groups. Null hypotheses of no difference were rejected if p-values were less than 0.05, or, equivalently, if the 95% confidence intervals (CIs) of risk point estimates excluded 1. All statistical analysis was performed using the IBM SPSS ver. 21.0 (IBM Co., Armonk, NY).
The study groups were well-matched, with male predominance and most patients falling within the 60- to 70-year age group. The commonest tumour site was the oesophagus(n=38) followed by stomach (n=30) and the gastrooesophageal junction (n=29). Most tumours were AC (n=86) with the remaining representing squamous cell carcinomas (n=10). There was evidence of positive HER-2 expression in two patients with GOJ AC.
The most common site of metastatic disease was nonregional lymphadenopathy followed by liver, peritoneum, lung, bone, and less common sites including adrenal glands, ovaries, and skin, with one instance of brain metastasis. The proportion of patients presenting with more than one site of metastatic disease ranged between 30%-50%. There was no significant difference in the frequency and distribution of different sites of metastatic disease between the two groups (Table 2).
Most patients (n=86) received triplet combination chemotherapy employing ECX (epirubicin, cisplatin, capecitabine), EOX (epirubicin, oxaliplatin, capecitabine) regime and nine patients received doublet chemotherapy with a platinum/FP regime. In addition, two patients with HER-2 positive tumours received a combination regime of platinum/FP and trastuzumab. The mean number of cycles received was similar in both groups and ranged from 5.84.±1.62 in group A to 5.45±1.53 in group B. All patients had responding or stable disease upon completion of chemotherapy except two patients in group A who developed mild disease progression characterised by progressive unilateral supraclavicular lymphadenopathy (vide infra). The proportion of patients receiving second line chemotherapy ranged between 35%-50% (Table 2).
Most patients received pRT to primary tumour as consolidation therapy within three months of completion of firstline pCT (n=44) with a median time-interval of 0.7 months (range, 0 to 2.9 months). In addition, nine patients received delayed pRT at the time of developing local tumour progression and more than 6 months after completion of first-line pCT with median time-interval of 10 months (range, 6 to 26 months). The palliative radiation dose-schedule of 30 Gy in 10 fractions was the most commonly employed radiationtherapy protocol in 38 patients (oesophagus and GOJ, n=28; gastric, n=10) followed by 20 Gy in five fractions in 13 patients (oesophagus and GOJ, n=9; gastric, n=4). In addition, two patients (GOJ, 1; gastric, 1) received 45 Gy in 25 fractions with concurrent chemotherapy.
All patients had stable and well-controlled metastatic disease at time of radiotherapy except two cases with progressive unilateral supraclavicular lymphadenopathy that was treated synchronously (20 Gy in 5 fractions) with pRT to the primary tumour. One patient with synchronous brain metastasis also received whole brain pRT (20 Gy in 5 fractions).
All patients completed radiotherapy as planned. Radiotherapy-induced esophagitis and nausea were common but no significant (grades 3/4) toxicities were observed except in one patient who required admission postradiotherapy with severe esophagitis requiring parenteral analgesia and nutritional support.
The median OS for the entire group was 16.9 months (range, 4.8 to 55.4 months). After median follow-up of 16.1 months (range, 4.8 to 55.4 months), 39/53 of patients (74%) had died in group A (pCT-RT) compared to 37/44 patients (84%) in group B (pCT). The median OS for patients treated with pCT-RT (group A) was 23.3 months (95% CI, 17.70 to 28.89 months) compared to 14 months (95% CI, 10.91 to 17.08 months) after pCT (group B) (p < 0.001). Similarly, the median PFS was significantly increased in patients treated with pCT-RT at 14 months (95% CI, 10.90 to 17.09 months) compared to 9.5 months (95% CI, 7.23 to 11.76 months) after pCT (p < 0.015) (Fig. 1). More specifically, the postradiotherapy OS in group A was 11.8 months (95% CI, 7.4 to 16.2 months) (Fig. 1).
We performed a multivariate analysis to assess the effect of different confounding variables on OS using Cox regression analysis and proportional hazard statistical model. The use of pCT-RT was the only independent positive predictor of survival. OS was not influenced by the site of tumour and the nature or distribution of metastatic disease (Fig. 2A). Furthermore, stratification analysis based on the above covariates demonstrated the presence of therapeutic benefit in favour of pCT-RT across all sites of tumour, including patients with gastric AC. The therapeutic benefit was observed in all subgroups of metastatic disease, although this was nonsignificant in patients with nonregional lymphadenopathy and liver metastasis (Fig. 2B).
Patients treated with higher biological equivalent dose (BED) dose-fractionation schedules demonstrated a trend towards improvement in OS. More specifically, patients treated with higher BED palliative regimes (30 Gy in 10 fractions or 45 Gy in 25 fractions) improved, although nonsignificantly, in OS, 26.2 months (95% CI, 21.5 to 30.9 months) compared to 16.0 months (95% CI, 9.58 to 22.41 months) in patients treated with lower BED regimes (20 Gy in 5 fractions) (p=0.08) (Fig. 2C).
Local recurrence (LR) was observed in 12/53 patients (23%) in group A (pCT-RT) compared to 16/44 patients (36%) in group B (pCT). All patients developing recurrence after pCT-RT underwent insertion of an oesophageal ELS as primary treatment for palliation of dysphagia. In comparison, 9/16 patients developing LR after pCT underwent insertion of ELS and 8/16 patients received pRT with one patient receiving combined stent and pRT. The median TTLP was significantly higher in patients after pCT-RT at 17.3 months (range, 5.23 to 44.50 months) compared to 8.3 months(range, 4.10 to 25.23 months) in patients treated with pCT (p=0.006).
In this retrospective study, we presented the therapeutic outcomes in metastatic OG cancer after a sequential multimodal approach in which patients responding well to initial chemotherapy were treated with pRT to primary tumour (Figs. 3 and 4). Most patients in our study received optimum schedules of chemotherapy. The median OS for the entire cohort of patients was 16.9 months, higher than previously reported historical controls. However, patients receiving pCT-RT improved significantly in PFS and OS compared to a favourable cohort of patients treated with chemotherapy alone. More specifically, patients treated with pCT-RT had an overwhelming survival benefit of approximately nine months, a new finding. The use of pCT-RT was associated with favourable response in all subgroups of metastatic disease, but the benefit was reduced and nonsignificant in patients with nonregional lymphadenopathy and liver metastasis. However, the observed survival benefit was robust and significant in patients with other visceral metastasis including peritoneum, bone and lung.
There were no objective assessments of QoL performed in the study, but patients treated with pCT-RT had reduced probability of local progression and significantly increased median TTLP compared to patients treated with chemotherapy alone. In addition, most patients maintained their nutritional status and general fitness/PS after pCT-RT which may reflect the higher proportion of patients receiving subsequent second-line therapies on relapse.
Most recent studies investigating pRT in advanced OG cancer reported its role for palliation of dysphagia in patients with or without ELS [8-13]. All of these studies consistently report successful and durable palliation of dysphagia after pRT, with a suggestion of superior outcomes in patients having combined ELS insertion and pRT (Table 3). However, the effects of pRT on survival are poorly defined, with a few studies reporting survival outcomes that are potentially contradictory and conflicting. Homs et al. [12] reported on a randomized phase III study comparing the outcomes of HDR-BT and ELS in which 209 patients were randomised to ELS placement (n=108) or single-dose (12 Gy) HDR-BT (n=101). Dysphagia improved more rapidly after ELS placement but long-term relief of dysphagia was better after HDR-BT combined with improved QoL measures. However, the use of HDR-BT was not associated with an improvement in OS. In contrast, some recent studies have reported on probable survival benefit in favour of pRT after ELS insertion compared to ELS alone [8-10]. These studies are limited by their predominantly retrospective nature and small sample size combined with heterogeneities across the study populations and therapeutic protocols. Sreedharan et al. [21] reported a large systematic review for the Cochrane database incorporating 2,542 patients from 40 studies and recommended HDR-BT as an appropriate alternative to ELS for palliation of dysphagia that may provide additional survival benefit with a better QoL. However, the role of pRT in metastatic gastric cancer is limited primarily to control of local bleeding complications [22,23]
There is limited evidence on the use of multi-modal therapy in metastatic OG cancer. Most initial studies evaluated the role of palliative conformal radiotherapy (CRT) primarily in patients with advanced oesophageal cancer, and demonstrated evidence of good local response ranging from 60%-90% but overall prognosis remained poor at approximately 6 months (Table 4) [14-20]. More recently, Ikeda et al. [20] reported on a retrospective study of 40 patients with metastatic SCC of the oesophagus treated with median four cycles (range, 1 to 7 cycles) of chemotherapy using 5 FU and cisplatin followed by concurrent CRT with 40 Gy in 20 fractions and two cycles of concurrent 5 FU and cisplatin. The study reported excellent local control of the primary lesion in 95% of patients including 12 patients (30%) who achieved a complete response. The median survival was 308 days, and the 1-year survival rate was 45%.
In contrast to the above studies, the multimodal approach adopted in our study supports the use of a sequential strategy in which patients developing good response to initial pCT received pRT to the primary tumour, but in the context of well-controlled metastatic disease. The limitations of a retrospective analysis are well-recognised including the existence of possible disease and treatment-related heterogeneities and selection bias. The observed heterogeneities in our study were not statistically significant and were not found to have any association with survival. However, this does not exclude the possibility of a selection bias particularly as there were no predefined clinical criteria for recommending pRT, which was essentially at the clinician’s discretion.
Approximately 90% of patients (86/97) in our series had AC, which are usually less sensitive to radiation than squamous cell cancers. Indeed, radical CRT is the widely accepted definitive therapeutic modality for localised squamous cell cancer of oesophagus. However, the effectiveness of combined-modality treatment in our cohort comprised primarily of AC reinforces the clinical importance of this therapeutic strategy and its possible application in the future. Furthermore, the benefit of palliative CRT was also observed in gastric AC that has not been reported previously.
The observed benefit after pCT-RT was more evident in patients receiving higher BED radiation dose-schedules indicative of possible dose-dependent effect. This remains untenable in the absence of rigid prescribing criteria and possibility of selection bias in determining appropriate doseradiation schedules. However, it does lend support for further investigation of alternative high-BED hypofractionated schedules (e.g., 40 Gy in 15 fractions; 36 Gy in 12 fractions) in future studies.
The possible intriguing cellular mechanisms by which pRT to primary tumour may influence metastatic disease remain unknown. However, the immunological effects of radiation are well-recognised including the existence of “abscopal” bystander effects that may influence tumour growth outside of the irradiated region. The development of bystander effect is proposed to be related to the activation of radiation-induced immunological cell-death mechanisms, as evident by an increase in levels of serum tumour necrosis factor after radiotherapy that can enhance the cytotoxic activity of natural killer cells [24,25].
The results from our study are encouraging and distinct, and provide indication of possible radiation-induced cellular mechanisms with systemic reverberations that may influence survival in patients with metastatic OG cancer. However, the true benefit of using this approach can be validated only in a randomized clinical trial that should also include investigation of alternative high-BED hypofractionated dose-schedules and objective assessment of other important parameters including QoL.
In our study, the median OS of patients who received pRT after initial chemotherapy was 23.3 months, one of the highest reported survival outcomes in metastatic OG cancer. Improved outcomes were observed when pRT was employed after optimum systemic dosage of chemotherapy and in the context of well-controlled metastatic disease. Furthermore, our study included patients with gastric cancer, and the use of multimodal therapy in metastatic gastric cancer has not been reported. The interesting and provocative observations in our study present strong arguments for further investigation of this strategy in randomized clinical trials.
References
1. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: esophageal and esophagogastric junction cancers [Internet]. Fort Washington, PA: National Comprehensive Cancer Network;2013. [cited 2014 Dec 1]. Available from: http://www.nccn.org.
2. Al-Batran SE, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008; 26:1435–42.

3. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–97.

4. Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009; 20:666–73.

5. Ross P, Nicolson M, Cunningham D, Valle J, Seymour M, Harper P, et al. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol. 2002; 20:1996–2004.

6. Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006; 24:4991–7.

7. Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008; 358:36–46.

8. Rueth NM, Shaw D, D'Cunha J, Cho C, Maddaus MA, Andrade RS. Esophageal stenting and radiotherapy: a multi-modal approach for the palliation of symptomatic malignant dysphagia. Ann Surg Oncol. 2012; 19:4223–8.

9. Eldeeb H, El-Hadaad HA. Radiotherapy versus stenting in treating malignant dysphagia. J Gastrointest Onco. 2012; 3:322–5.
10. Zhong J, Wu Y, Xu Z, Liu X, Xu B, Zhai Z. Treatment of medium and late stage esophageal carcinoma with combined endoscopic metal stenting and radiotherapy. Chin Med J (Engl). 2003; 116:24–8.
11. Hanna WC, Sudarshan M, Roberge D, David M, Waschke KA, Mayrand S, et al. What is the optimal management of dysphagia in metastatic esophageal cancer? Curr Oncol. 2012; 19:e60–6.

12. Homs MY, Steyerberg EW, Eijkenboom WM, Tilanus HW, Stalpers LJ, Bartelsman JF, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet. 2004; 364:1497–504.

13. Sharma V, Mahantshetty U, Dinshaw KA, Deshpande R, Sharma S. Palliation of advanced/recurrent esophageal carcinoma with high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 2002; 52:310–5.

14. Coia LR, Soffen EM, Schultheiss TE, Martin EE, Hanks GE. Swallowing function in patients with esophageal cancer treated with concurrent radiation and chemotherapy. Cancer. 1993; 71:281–6.

15. Urba SG, Turrisi AT 3rd. Split-course accelerated radiation therapy combined with carboplatin and 5-fluorouracil for palliation of metastatic or unresectable carcinoma of the esophagus. Cancer. 1995; 75:435–9.

16. Hayter CR, Huff-Winters C, Paszat L, Youssef YM, Shelley WE, Schulze K. A prospective trial of short-course radiotherapy plus chemotherapy for palliation of dysphagia from advanced esophageal cancer. Radiother Oncol. 2000; 56:329–33.

17. Harvey JA, Bessell JR, Beller E, Thomas J, Gotley DC, Burmeister BH, et al. Chemoradiation therapy is effective for the palliative treatment of malignant dysphagia. Dis Esophagus. 2004; 17:260–5.

18. Burmeister BH, Walpole ET, Burmeister EA, Thomas J, Thomson DB, Harvey JA, et al. Feasibility of chemoradiation therapy with protracted infusion of 5-fluorouracil for esophageal cancer patients not suitable for cisplatin. Int J Clin Oncol. 2005; 10:256–61.
19. Cho SH, Shim HJ, Lee SR, Ahn JS, Yang DH, Kim YK, et al. Concurrent chemoradiotherapy with S-1 and cisplatin in advanced esophageal cancer. Dis Esophagus. 2008; 21:697–703.

20. Ikeda E, Kojima T, Kaneko K, Minashi K, Onozawa M, Nihei K, et al. Efficacy of concurrent chemoradiotherapy as a palliative treatment in stage IVB esophageal cancer patients with dysphagia. Jpn J Clin Oncol. 2011; 41:964–72.

21. Sreedharan A, Harris K, Crellin A, Forman D, Everett SM. Interventions for dysphagia in oesophageal cancer. Cochrane Database Syst Rev. 2009; (4):CD005048.

22. Asakura H, Hashimoto T, Harada H, Mizumoto M, Furutani K, Hasuike N, et al. Palliative radiotherapy for bleeding from advanced gastric cancer: is a schedule of 30 Gy in 10 fractions adequate? J Cancer Res Clin Oncol. 2011; 137:125–30.
23. Lee JA, Lim DH, Park W, Ahn YC, Huh SJ. Radiation therapy for gastric cancer bleeding. Tumori. 2009; 95:726–30.

Fig. 1.
(A) Schematic illustration of study design. (B) Palliative radiotherapy after initial palliative chemotherapy (pCT-RT) prolongs progression-free survival (PFS) and overall survival (OS) compared to palliative chemotherapy (pCT) alone. The median PFS of patients treated with pCT-RT was significantly increased at 14 months compared to 9.5 months in patients treated with pCT (p < 0.015). The median OS of patients after pCT-RT was 23.3 months compared to 14 months in patients treated with pCT alone (p < 0.001). OG, esophago-gastric.
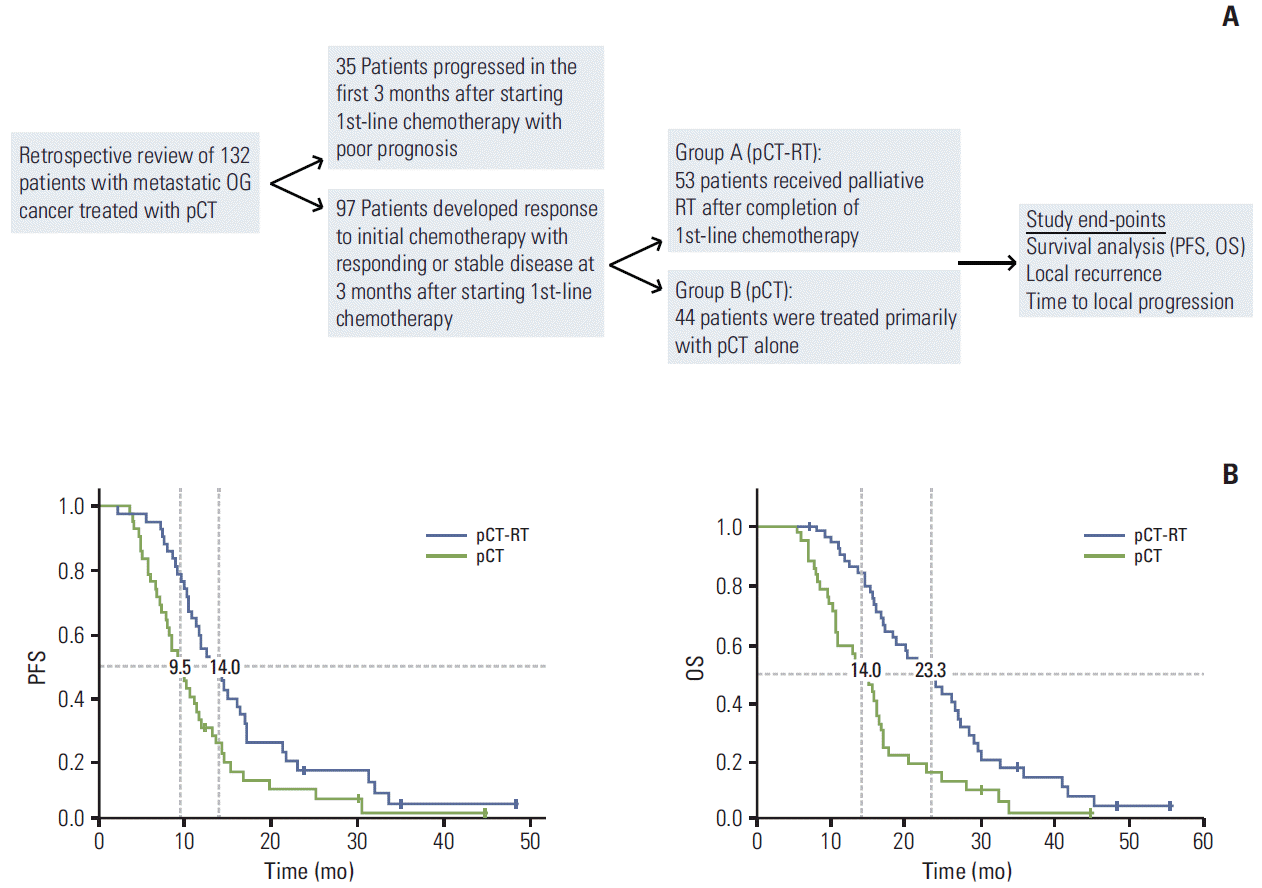
Fig. 2.
Effect of different variables on survival outcomes. (A) The use of pCT-RT was an independent predictor of overall survival on multivariate analysis that was not influenced by the site of tumour or nature of metastatic disease. (B) The survival benefit of pCT-RT was observed in all sub-groups independent of site and number of metastasis and subsequent lines of systemic chemotherapy. (C) There was favorable trend with increased survival benefit (non-significant) in patients treated with higher biological equivalent dose of radiation (p=0.08). HR, hazard ratio; CI, confidence interval; GOJ, gastro-esophageal junction; pCT-RT, palliative chemotherapy-radiotherapy.
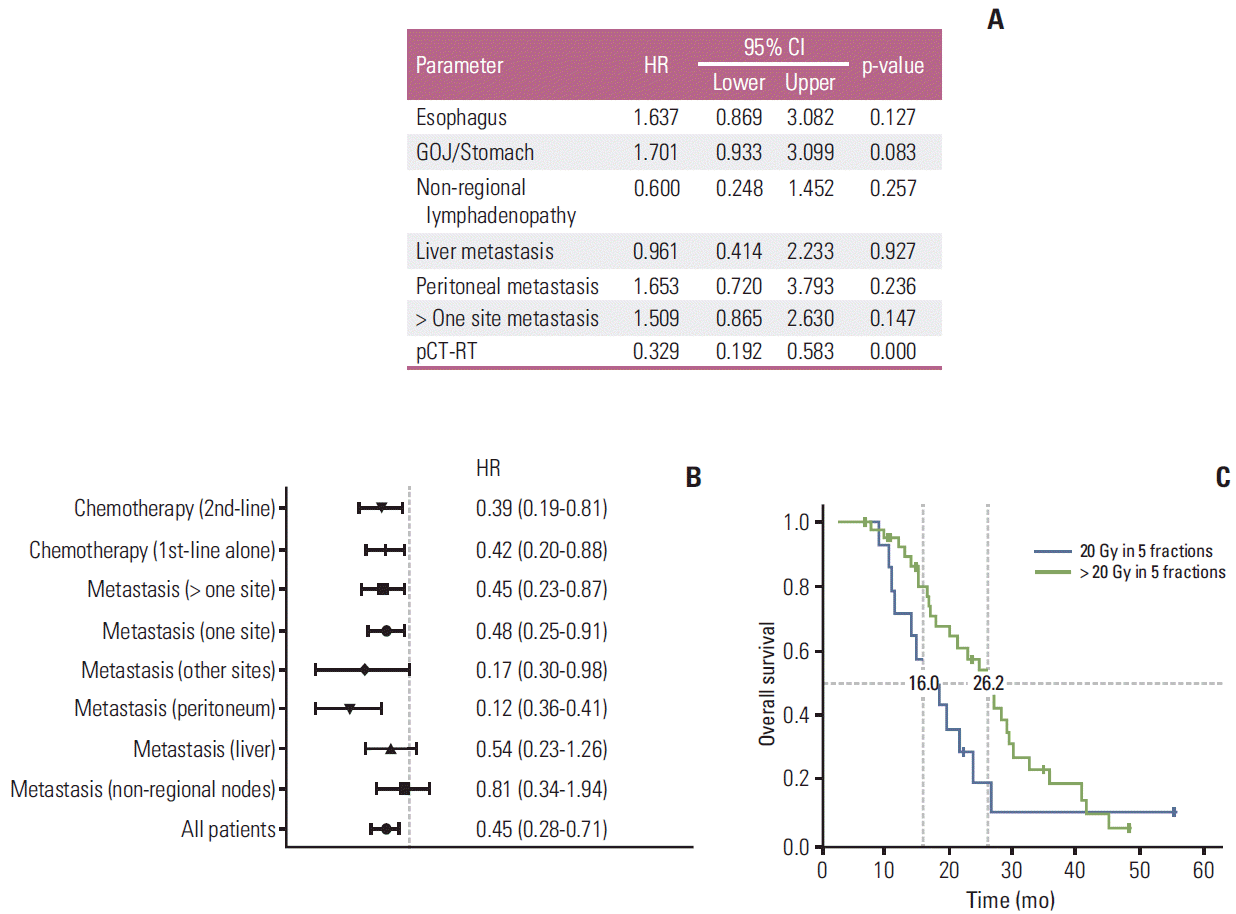
Fig. 3.
Case examples of diagnosis and multi-modality management of cancers of esophagus. (A) A 50-year-old female; cancer of lower esophagus and GOJ (arrow) (AC); liver and brain metastasis (arrow); 8 cycles of EOX chemotherapy; palliative radiotherapy to whole brain and primary tumor (20 Gy in 5 fractions); OS of 11 months (dead). (B) A 48-year-old male; cancer of esophagus and GOJ (AC); enlarged FDG-avid coeliac (yellow arrow) and para-aortic lymphadenopathy (red arrow) with lung metastasis; 6 cycles of EOX chemotherapy; palliative radiotherapy to primary tumor (30 Gy in 10 fractions); OS of 35 months (alive). GOJ, gastro-esophageal junction; AC, adenocarcinoma; EOX, epirubicin, oxaliplatin, capecitabine; OS, overall survival.
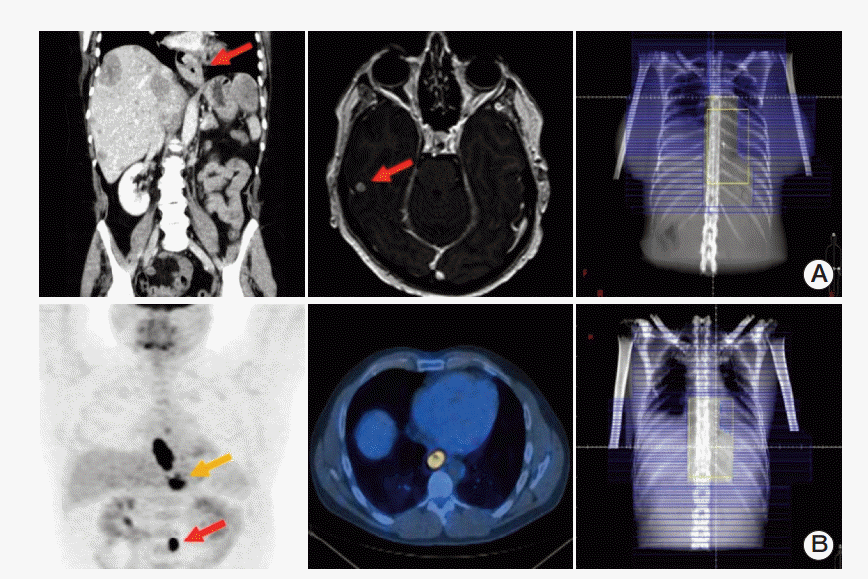
Fig. 4.
Case examples of diagnosis and multi-modality management of cancers of stomach. (A) A 61-year-old male; cancer of stomach (AC); locally advanced disease at pylorus with contiguous involvement of left lobe of liver (arrow); liver and lung metastasis; 6 cycles of EOX chemotherapy; palliative radiotherapy to primary tumor (20 Gy in 5 fractions); OS of 20 months (dead). (B) A 75-year-old male; cancer of stomach (AC); locally advanced disease at linitis plastica (arrow); omental disease; 6 cycles of EOX chemotherapy; palliative radiotherapy to primary tumor (30 Gy in 10 fractions); OS of 12.4 months (dead). AC, adenocarcinoma; EOX, epirubicin, oxaliplatin, capecitabine; OS, overall survival.
Table 1.
Summary of radiotherapy characteristics for patients in group A (pCT-RT)
| Characteristic | No. |
|---|---|
| Time-interval after lst-line chemotherapy (mo) | |
| Consolidation radiotherapy (n=44) | |
| Mean±SD | 1±0.72 |
| Median (range) | 0.7 (0-2.9) |
| Delayed radiotherapy (n=9) | 10 (6-26) |
| Irradiated tumour site | |
| Esophagus and GOJ | 38 |
| Gastric | 15 |
| Synchronous metastasis | |
| Supraclavicular lymphadenopathy | 2 |
| Brain | 1 |
| Dose and fractionation | |
| 20 Gy in 5 fractions | |
| Esophagus and GOJ | 9 |
| Gastric | 4 |
| 30 Gy in 10 fractions | |
| Esophagus and GOJ | 28 |
| Gastric | 10 |
| 45 Gy in 25 fractions | |
| GOJ | 1 |
| Gastric | 1 |
| RT technology and planning | |
| Esophagus and GOJ/gastric (20 Gy in 5 fractions)a) | |
| Median field size (cm) | (13.8±3.23)x(11.5±2.84) |
| Gastric (30 Gy in 10 fractions)b) | |
| Median PTV volume (range, cm3) | 820 (192-2,256) |
| Dosimetry, median dose (range, Gy) | |
| 20 Gy in 5 fractions | 20.50±0.45 (19.5±0.15-22.2±0.69) |
| 30 Gy in 10 fractions | 30.40±0.35 (27±2.47-32.5±5.5) |
All patients were treated with palliative external beam radiotherapy after initial chemotherapy in the presence of responding or stable metastatic disease. pCT-RT, palliative chemotherapy-radiotherapy; SD, standard deviation; GOJ, gastro-esophageal junction.
Table 2.
Patient demographics, disease, and treatment (chemotherapy)-related characteristics
Values are presented as number (%) unless otherwise indicated. pCT-RT, palliative chemotherapy-radiotherapy; SD, standard deviation; GOJ, gastro-esophageal junction; AC, adenocarcinoma; SCC, squamous cell cancer; EOX, epirubicin, oxaliplatin, capecitabine; ECX, epirubicin, cisplatin, capecitabine; FP, fluoropyrimidine.
Table 3.
Summary of outcomes from studies of palliative radiotherapy in metastatic esophago-gastric cancer
| Source | Study design | Study protocol | Outcome |
|---|---|---|---|
| Rueth et al. (2012) [8] |
Retrospective Assessment of effects of pRT on symptoms and OS in patients with cancer of esophagus after ELS insertion |
45 Patients All patients had ELS insertion 25 Patients received pRT |
Subjective improvement in dysphagia after ELS in 68.9% No symptom benefit with addition of pRT 30-Day mortality 15.6% pRT was associated with increased MS compared with ELS alone (98 days vs. 38 days) |
| Hanna et al. (2012) [11] |
Retrospective Assessment of effects of pRT and ELS on symptoms in patients with cancer of esophagus |
63 Patients 50/63 (79%) received pRT (HDR-BT in 18/50, EBRT in 10/50, EBRT+BT in 22/50) 13/63 (21%) underwent ELS insertion |
Mean wait time from diagnosis to treatment: stent (22 days) and RT (54 days) (p=0.003) 85% with stent were palliated within 2 weeks compared with 50% after pRT Symptoms recurred after stent in 20% at 10 weeks compared with 10% after RT |
| Eldeeb and El-Hadaad (2012) [9] |
Prospective Assessment of effects of pRT±ELS on symptoms and OS in patients with cancer of esophagus |
91 Patients pRT (n=30), ELS (n=35), pRT+ELS (n=26) |
ELS was associated with rapid palliation of symptoms compared with pRT alone Median OS significantly increased in ELS+RT (237 days) compared with pRT (119 days) and ELS (169 days) (p=0.01) |
| Homs et al. (2004) [12] |
Randomized phase III RCT to assess and compare the effects of HDR-BT compared with ELS for palliation of dysphagia in patients with cancer of esophagus |
209 Patients HDR-BT (n=108), ELS (n=101) |
Rapid improvement of dysphagia after ELS compared with HDR-BT Increased duration of palliation after HDR-BT No differences in OS but QoL better after HDR-BT |
| Zhong et al. (2003) [10] |
Prospective Assessment of effects of pRT and ELS on symptoms and OS in patients with cancer of esophagus |
34 Patients ELS (n=18), ELS+pRT (n=16) |
ELS+pRT was associated with improvement in OS (37.5%) compared with ELS (11%) at 12 months (p < 0.01) Significant reduction in dysphagia recurrence after ELS+RT (p < 0.01) |
| Sharma et al. (2002) [13] |
Retrospective Assessment of the effects of pRT on symptoms and OS in patients with cancer of esophagus |
64 Patients HDR-BT (n=38), HDR-BT+pRT (n=20) |
Dysphagia improved in 38% and stabilized in 41% of patients Median dysphagia-free survival of 10 months Median OS of 7 months |
Table 4.
Summary of outcomes from studies of multimodality therapy in advanced esophagus-gastric cancer
| Source | Study design | Treatment protocol | Outcome |
|---|---|---|---|
| Coia et al. (1993) [14] | Retrospective | 49 Patients | Local response in 91% patients |
| SCC and AC | pRT (50 Gy in 25 fractions) with concurrent chemotherapy (5 FU and mitomycin) | Median OS of 8 months | |
| Urba and Turrisi (1995) [15] | Retrospective | 27 Patients | Local response in 17/27 (59%) |
| AC 75%, SCC 25% | pRT with concurrent carboplatin and 5 FU | Median OS of 6 months | |
| Hayter et al. (2000) [16] | Prospective | 22 Patients | Local response in 15/22 (68%) |
| Phase I/II study | pRT (30 Gy in 10 fractions) with concurrent single course of chemotherapy (5 FU and mitomycin) | Median OS of 5 months | |
| SCC and AC | |||
| Harvey et al. (2004) [17] | Retrospective | 106 Patients | Local response in 78% |
| SCC and AC | pRT (35 Gy in 15 fractions) with concurrent single course of 5-FU-based chemotherapy | Median OS of 7 months | |
| Burmeister et al. (2005) [18] | Retrospective | 24 Patients | Local response in 67% |
| SCC and AC | pRT (35 Gy in 15 fractions) with concurrent continuous infusion of 5 FU | Median OS of 9 months | |
| Cho et al. (2008) [19] | Retrospective | 37 Patients | Local response in 76% |
| SCC | pRT (54 Gy in 27 fractions) with concurrent S-1 and cisplatin | Median OS of 11.6 months | |
| Further 6 cycles of S-1/cisplatin | |||
| Ikeda et al. (2011) [20] | Retrospective | 40 Patients | Local response in 95% |
| SCC | Initial chemotherapy with 5 FU and cisplatin | Median OS of 12 months | |
| 40 Gy in 20 fractions combined with concurrent 5 FU and cisplatin |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download