Abstract
Purpose
The purpose of this study was to evaluate whether an exogenous epidermal growth factor (EGF) could induce anti-tumor and radiosensitizing effects in vivo.
Materials and Methods
BALB/c-nu mice that were inoculated with A431 (human squamous cell carcinoma) cells in the right hind legs were divided into five groups: I (no treatment), II (EGF for 6 days), III (EGF for 20 days), IV (radiotherapy [RT]), and V (RT plus concomitant EGF). EGF was administered intraperitoneally (5 mg/kg) once a day and the RT dose was 30 Gy in six fractions. Hematoxylin and eosin (H&E) stained sections of tumor, liver, lung, and kidney tissues were investigated. Additionally, tumors were subjected to immunohistochemistry staining with caspase-3.
Results
EGF for 6 days decreased tumor volume, but it approached the level of the control group at the end of follow-up (p=0.550). The duration of tumor shrinkage was prolonged in group V while the slope of tumor re-growth phase was steeper in group IV (p=0.034). EGF for 20 days decreased tumor volume until the end of the observation period (p < 0.001). Immunohistochemistry revealed that mice in group V showed stronger intensity than those in group IV. There were no abnormal histological findings upon H&E staining of the normal organs.
Epidermal growth factor (EGF) is a single polypeptide of 53 amino acid residues that is involved in cell growth, proliferation, and differentiation [1]. EGF stimulates target cells by binding to epidermal growth factor receptor (EGFR), which is a transmembrane receptor with intrinsic tyrosine kinase activity. The interaction of EGF-EGFR induces dimerization and autophosphorylation of the receptor, while regulating the expression level of a variety of transcription factors through multiple signaling pathways [2].
Contrary to the conventional concept of EGF action, treatment with EGF is sometimes associated with increased cell death. Cao et al. [3] demonstrated that the nanomole level of EGF decreased cell adhesion and induced apoptosis in the A431 (human squamous cell carcinoma) cell line, and EGF was shown to induce apoptosis related to cell cycle arrest at the G1 check point with alterations in expression levels of regulatory proteins. In addition, EGF binding leads to the internalization of EGFR, and this structural modification is known to be a specified process of EGF-induced apoptosis [4,5]. More recently, studies have discussed the endocytoplasmic regulation of the EGF-EGFR complex and its association with signal transducer and activator of transcription-1 (STAT-1) and p38 mitogen-activated protein kinase (MAPK) [6-8].
Targeting agents of the EGFR-related signaling pathway have been proposed as a novel treatment modality [9]. The inhibition of EGFR signaling pathways was shown to induce cytostatic and cytotoxic effects and decrease repopulation of tumor cells [10]. Moreover, preclinical and clinical studies of the combined use of EGFR inhibitors and radiotherapy (RT) have indicated that the inhibition of EGFR enhanced therapeutic efficacy of fractionated irradiation [11,12]. Based on these principles, the present study focused on contradictory concepts, EGF-induced anti-tumor effect and radiosensitization. Although previous investigations reported in vitro results of enhanced radioresponse by EGF, few studies have evaluated this in vivo [13-15].
Therefore, this study was conducted to evaluate the tumor growth suppression and radiation-sensitizing effects of EGF in A431 xenograft tumor models. Potential adverse systemic effects of EGF on major normal organs were also examined.
This experiment was approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University Hospital (IACUC approval No. 12-0179). The National Research Council guidelines for the care and use of laboratory animals were observed. Male 5-week-old BALB/c-nu mice were used in this study. The animals were maintained at the Biomedical Research Institute of Seoul National University Hospital, which was approved by the Korean Food and Drug Administration and complies with the regulations and standards of the IACUC of Seoul National University Hospital. The mice were housed under pathogen-free conditions with controlled humidity (40%-60%) and temperature (20°C-24°C). Animals were housed under a 12-hour light/dark cycle, with lights on from 8 AM to 8 PM The mice were kept in an individual ventilated cage system on sawdust bedding. Standard mouse diet and filtered city tap water from standard Perspex drinking bottles were provided ad libitum.
We used A431 cells that originated from human vulvar epidermoid carcinoma. The cell line was purchased from the American Type Culture Collection (Manassas, VA), and cells were maintained in Dulbecco's modified Eagle's medium (Gibco BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (Gibco BRL). Cells were grown in an incubator with a humidified atmosphere of 95% air/5% CO2 at 37.5°C.
Recombinant human EGF was provided by Daewoong Pharmaceutical Company (Seoul, Korea) in the form of powder. The agent was diluted in phosphate-buffered saline at a concentration of 1 mg/mL and stored at –70°C.
A total of 5×105 A431 cells were injected subcutaneously into the right hind leg of the mice. When the tumors reached a volume of 200 mm3 at about 7-9 days after the inoculation, mice were divided into five groups (n=8 for each group). An additional two mice (n=2) that were not inoculated with tumor cells received intraperitoneal EGF for 20 days in each experiment. The animals were monitored for 6 months without other interventions to evaluate potential adverse effects of EGF on major organs. Fig. 1 illustrates the treatment design of the five experimental groups. The control group (group I) received no treatment, while the others received EGF for 6 days, EGF for 20 days, RT (30 Gy/6 fractions [fx], daily), and RT (30 Gy/6 fx, daily) plus concomitant EGF (for 6 days) (groups II, III, IV, and V, respectively) (Fig. 1). EGF was administered by intraperitoneal injection (5 mg/kg) once a day. The injection dose was determined by considering the half-life of the drug, and the feasibility was examined in the preliminary experiments. RT was delivered using 6-MV photon energy (Clinac 6/100, Varian Medical Systems, Palo Alto, CA). The fraction size was 5 Gy/fx and the total RT dose was 30 Gy. A custom-made acrylic device was employed to immobilize the body and the leg tumors. In the RT plus EGF group (group V), EGF was injected several minutes before irradiation. A time interval existed due to positioning of mice on the treatment couch, opening/closing the door of the treatment room, and the beam-on time with manipulation of the treatment machine. Day 1 was defined as the start date of each treatment.
Tumor size was measured every other day using a Vernier caliper by two independent researchers (Y.J.L. and S.-R.J.) until day 23. To determine a humane endpoint, the entire period of observation was stopped before the maximum diameter of a single tumor exceeded 2 cm. Tumor volume was calculated according to the formula 1/2×length×width2 (mm3). Mice were sacrificed on days 0, 12, and 23 to obtain paraffin blocks of tumor tissues and major organs, such as liver, lung, and kidney. The relative tumor volume was defined as the ratio between the final volume and the initial volume. The experiments were independently repeated three times.
Five-micrometer-thick, paraffin-embedded tumor sections were cut and deparaffinized in Dako PT Link (Dako North America Inc., Carpinteria, CA) and then stained with hematoxylin and eosin (H&E). For immunohistochemical staining, the antigen retrieval process was performed at 97°C using target retrieval solution. Slides were rinsed with Envision FLEX Wash Buffer (Dako North America Inc.) and washed with diluted water. Endogenous blocking with 3% H2O2 was performed for 5 minutes. The primary antibodies, anti-EGFR (1:50, #4267, Cell Signaling, Danvers, MA) and anti-cleaved caspase-3 (1:50, #9661, Cell Signaling) were diluted with antibody diluents (Invitrogen Life Technologies, Carlsbad, CA). Buffer solution was used again, and secondary antibodies of horseradish peroxidase-labeled polymer anti-rabbit were applied. The samples were developed with Dako REAL 3,3'-diaminobenzidine+chromogen (Dako North America Inc.) and treated with Mayer’s hematoxylin. After multi-step dehydration with 95% and 100% alcohol, xylene was used to remove the alcohol.
H&E-stained slides of tumor and major organ tissues of groups I-V were examined. The morphological findings were then analyzed to assess the systemic impact of exogenous EGF. Upon immunohistochemistry analysis, the expression level of EGFR in A431 cells was evaluated, and cleaved caspase-3 was used to analyze EGF-induced apoptosis in tumor tissues. A single pathologist (J.M.K.) reviewed the immunohistochemistry results without prior knowledge of treatment outcome. The intensity of expression was reported in a semi-quantitative manner.
Data were presented as the mean±standard deviation of the relative tumor volume. The mean data of three independent experiments were included. Since the tumor volumes were characterized as longitudinal data with repeated measurements, the differences among the experimental groups were analyzed by linear mixed model analysis. Groups I-III were analyzed together to evaluate the EGF-induced tumor growth suppression, and groups IV and V were compared to investigate enhancement of the radioresponse. The results were considered to be statistically significant if the p-value was less than 0.05. All analyses were performed using SPSS ver. 20.0 (IBM Co., Armonk, NY).
Figs. 2 and 3 show the relative tumor volumes of all experimental groups. Although mice treated with EGF for 6 days showed decreased tumor growth relative to the control group (Fig. 2), the tumor volume of group II (EGF×6) approached the level of the control group on day 23 (p=0.550). In the RT and RT with concomitant EGF for 6 days groups (groups IV and V, respectively), differences in relative tumor volume were observed after emergence of the first minimal value (Fig. 2). Group IV (RT) showed the minimal value on day 9 (0.92±0.05), while the tumor volume of group V (RT+EGF) decreased continuously until day 13 (0.69±0.11). After the minimal points, relative tumor volumes increased in both group IV (RT) and V (RT+EGF). The slope of the tumor re-growth phase was significantly steeper in group IV than V throughout the follow-up period.
Fig. 3 shows differences in relative tumor volume among groups I (no treatment), II (EGF×6), and III (EGF×20). In contrast to group II (EGF×6), the anti-tumor effect of EGF for 20 days was maintained until the end of the follow-up period. On day 23, the mean relative tumor volumes of group I (no treatment), II (EGF×6), and III (EGF×20) were 17.63, 16.48, and 10.64, respectively. The tumor volume of group III (EGF×20) was significantly lower than that of group I (no treatment) and II (EGF×6; p < 0.001 for both comparisons).
The immunohistochemistry of the cleaved caspase-3 antibody revealed EGF-induced apoptosis in the tumor tissues. The staining intensity of each group is listed in Table 2. The apoptotic activity of cleaved caspase-3 was defined as the proportion of apoptotic cells in the tumor sections on each slide (day 12). Cytoplasmic uptake of the antibody was stronger in groups II (EGF×6) and III (EGF×20) than in group I (no treatment). The immunostaining intensity was also higher in group V (RT+EGF) than in group IV (RT) (Fig. 5).
No abnormal histological findings were observed on H&E slides of the liver, lung, and kidney of sacrificed mice that had been treated with EGF (Fig. 6). Liver sections showed normal hepatic lobules and portal areas with intact perilobular and intralobular systems. In lung sections, normal bronchiolar and alveolar structures were observed, while no abnormalities were observed in the glomerulus, capillary, or renal ductal structures in kidney sections. Evaluation of the additional mice treated with EGF for 20 days in the absence of tumor inoculation revealed no death events, and no abnormal histologic features were observed in major normal organs during the 6 month follow-up period.
The anti-tumor effects of exogenous EGF have been known for decades. However, most previous investigations of these effects were based on in vitro experiments. Therefore, this study was conducted to establish EGF-induced cell death in mouse xenograft models using A431 cells and its potential as a radiation sensitizer in the design of fractionated irradiation.
The growth curve of the control group was steeper than that of groups II (EGF×6) and III (EGF×20), and the final tumor volume of untreated mice was significantly higher at the end of follow-up. However, the tumors of groups II (EGF×6) and III (EGF×20) also continued to grow, and no absolute decrease in tumor volume was observed over the entire follow-up period. The injection of exogenous EGF suppressed tumor growth, but was not clearly associated with tumor shrinkage.
Choi et al. [17] evaluated the anti-tumor effects of EGF in xenograft mouse models. Their study compared potential cytotoxic effects of EGF (intraperitoneal, 1 mg/kg, every other day, three times) and cisplatin (intraperitoneal, 5 mg/kg, once) using subcutaneously inoculated human cancer cell lines, including A431 cells. Although the study design differed from that of the present study, the patterns of growth suppression were similar to those in the present study. The authors also demonstrated the possibility of EGF as a cytotoxic agent in certain types of tumors.
Intraperitoneal injection of EGF for 6 days did not lead to a statistically significant difference in tumor size compared to the control group, indicating that the treatment did not exert an anti-tumor effect. However, the concomitant EGF (for 6 days) with fractionated RT (30 Gy in 6 fx) resulted in more prolongation of the therapeutic effect and slower tumor re-growth relative to the RT-only group. These findings suggest that the concomitant use of EGF enhanced the radioresponse during treatment and decreased tumor repopulation after completion of RT.
The current findings are in agreement with previous in vitro results. Kwok and Sutherland [13] initially reported that an exogenous EGF after or continuously before, during, and after irradiation enhanced the radiosensitivity of human squamous cell carcinoma cell lines in vitro. They demonstrated that EGF enhanced radiosensitivity with reduction in the shoulder region of the cell survival curves, and that the maximum effect was observed in response to treatment with 10 ng/mL of EGF. In addition, the EGF-related enhancement of irradiation was higher in the G1 phase with a wider shoulder region than other phases [14,18]. However, few studies have reported the impact of EGF on radiosensitivity to date.
Kwon et al. [19] recently designed in vitro and in vivo studies to evaluate the effects of EGF on cell proliferation and radiation survival. In their study, a clonogenic assay revealed that EGF suppressed tumor growth in human cancer cell lines with high expression of EGFR, and that the inhibitory effect was more evident at higher concentrations of EGF (1 nM or more). When EGF was combined with irradiation in vitro, EGF enhanced the cell killing effect in cancer cell lines, but not in normal fibroblast cells. In an in vivo study of EMT-6 (mouse mammary sarcoma), treatment with EGF (1 mg/kg for 7 consecutive days, three times a day) decreased tumor volume, but this decrease was not statistically significant. The authors concluded that further studies were needed to investigate such effects in human cancer cell lines.
It should also be noted that overall growth inhibition was more evident in group III (EGF×20) than in group II (EGF×6). Although the tumor volume of mice treated with EGF for 6 days eventually approached the level of the control group, the anti-tumor effect of EGF for 20 days was maintained. Given that a longer duration of daily EGF injections induced more tumor suppression, we suggest that adequate exposure to EGF is needed to obtain an observable level of EGFinduced tumor suppression. However, since the mice treated with EGF for 20 days were sacrificed on the third day following completion of the treatment, there is a possibility of delayed tumor growth. Accordingly, further studies are needed to evaluate the differential kinetics based on total duration of EGF treatment in vivo.
Caspase-3 is one of the key molecules of the apoptosis pathway. This study provided histologic evidence of increased apoptotic cells in mice treated with RT+EGF, which was correlated with increased radioresponse and smaller tumor volumes. Song et al. [4] performed an in vitro study with A431 cells and found that sustained activation of EGFR by EGF was related to activation of caspase-3 and a time-dependent cleavage of caspase-8 from protease assay and western blot analysis. They concluded that EGF-mediated apoptosis could be induced by both mitochondrial and non-mitochondrial pathways. In addition, Chiu et al. [20] conducted an in vitro study using neuroblastoma cell lines and demonstrated that the maximum level of total caspase-3 expression could be obtained from incubation with EGF (5 ng/mL).
In the present study, the impact of EGF on major normal organs was evaluated, and the intraperitoneal injection of EGF did not induce adverse histological changes in the liver, lung, or kidney. Furthermore, extra mice treated with EGF for 20 days without tumor inoculation survived for 6 months without abnormal histological findings. Therefore, it was suggested that intraperitoneal EGF induced little systemic toxicity in the major organs studied. However, the absence of a histologic reaction does not guarantee clinical safety of the treatment because this preclinical study is based on immunosuppressed mice. Careful consideration should be given to different host factors, such as innate immunity and pharmacokinetics of intraperitoneal EGF. Potential systemic impact on other organs, including the intestine and the brain, must also be evaluated.
Several mechanisms of EGF-induced apoptosis have been proposed. In previous studies, p21-dependent arrest in the G1 phase was considered an important principle [21,22]. Cao et al. [3] also reported that EGF treatment increased p21, but suppressed cyclin A and D1, cyclin-dependent kinase 2, and phosphorylated retinoblastoma protein, which induced cell cycle arrest and decline in cell adhesion. In addition, the role of STAT-1, a downstream molecule in the EGFR pathway, was substantiated [8,23]. More recently, several studies demonstrated that p38 MAPK mediates STAT-1 tyrosine phosphorylation, which leads to the apoptotic response of an exogenous EGF [4,7]. However, little is known about the potential mode of action of the radiosensitizing effect of EGF. In earlier in vitro studies, the enhancement of radiation sensitivity was more prominent in cancer cells with higher EGFR expression [14]. Furthermore, cell-cycle dependency was observed, and the G1 phase was found to be most responsive [18].
This study was designed to determine whether the paradoxical in vitro effects of EGF-induced cell death in A431 cells are also reproducible in mouse xenograft models. Although further in vitro analyses are needed to elucidate the underlying mechanism, the results of the present study verified the anti-tumor effect of exogenous EGF in vivo and demonstrated the potential role of EGF as a radiation sensitizer. Beyond the original knowledge of increased tumor cell proliferation and pro-survival effect of EGF-EGFR interaction, a new concept of EGF-induced tumor suppression was remarkable. To the best of the author’s knowledge, this is the first in vivo study to investigate the combined use of EGF with RT and its radiosensitizing effect in the design of fractionated irradiation. Further studies are needed to generalize these results in other cell lines and evaluate the feasibility of EGF treatment.
ACKNOWLEDGMENTS
This work was supported by a grant from the Korea Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A120313), and the National R&D program through the Dong-nam Institute of Radiological & Medical Sciences (DIRAMS) funded by the Ministry of Education, Science and Technology (50595-2013).
References
1. Boonstra J, Rijken P, Humbel B, Cremers F, Verkleij A, van Bergen en Henegouwen P. The epidermal growth factor. Cell Biol Int. 1995; 19:413–30.
2. Henson ES, Gibson SB. Surviving cell death through epidermal growth factor (EGF) signal transduction pathways: implications for cancer therapy. Cell Signal. 2006; 18:2089–97.

3. Cao L, Yao Y, Lee V, Kiani C, Spaner D, Lin Z, et al. Epidermal growth factor induces cell cycle arrest and apoptosis of squamous carcinoma cells through reduction of cell adhesion. J Cell Biochem. 2000; 77:569–83.

4. Song JY, Lee SW, Hong JP, Chang SE, Choe H, Choi J. Epidermal growth factor competes with EGF receptor inhibitors to induce cell death in EGFR-overexpressing tumor cells. Cancer Lett. 2009; 283:135–42.

5. Worthylake R, Wiley HS. Structural aspects of the epidermal growth factor receptor required for transmodulation of erbB-2/neu. J Biol Chem. 1997; 272:8594–601.

6. Rush JS, Quinalty LM, Engelman L, Sherry DM, Ceresa BP. Endosomal accumulation of the activated epidermal growth factor receptor (EGFR) induces apoptosis. J Biol Chem. 2012; 287:712–22.

7. Kozyulina PY, Okorokova LS, Nikolsky NN, Grudinkin PS. p38 MAP kinase enhances EGF-induced apoptosis in A431 carcinoma cells by promoting tyrosine phosphorylation of STAT1. Biochem Biophys Res Commun. 2013; 430:331–5.

8. Grudinkin PS, Zenin VV, Kropotov AV, Dorosh VN, Nikolsky NN. EGF-induced apoptosis in A431 cells is dependent on STAT1, but not on STAT3. Eur J Cell Biol. 2007; 86:591–603.

10. Nyati MK, Morgan MA, Feng FY, Lawrence TS. Integration of EGFR inhibitors with radiochemotherapy. Nat Rev Cancer. 2006; 6:876–85.

11. Huang SM, Harari PM. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin Cancer Res. 2000; 6:2166–74.
12. Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010; 11:21–8.

13. Kwok TT, Sutherland RM. Enhancement of sensitivity of human squamous carcinoma cells to radiation by epidermal growth factor. J Natl Cancer Inst. 1989; 81:1020–4.

14. Kwok TT, Sutherland RM. Differences in EGF related radiosensitisation of human squamous carcinoma cells with high and low numbers of EGF receptors. Br J Cancer. 1991; 64:251–4.

15. Bonner JA, Maihle NJ, Folven BR, Christianson TJ, Spain K. The interaction of epidermal growth factor and radiation in human head and neck squamous cell carcinoma cell lines with vastly different radiosensitivities. Int J Radiat Oncol Biol Phys. 1994; 29:243–7.

16. Licitra L, Perrone F, Tamborini E, Bertola L, Ghirelli C, Negri T, et al. Role of EGFR family receptors in proliferation of squamous carcinoma cells induced by wound healing fluids of head and neck cancer patients. Ann Oncol. 2011; 22:1886–93.

17. Choi J, Moon SY, Hong JP, Song JY, Oh KT, Lee SW. Epidermal growth factor induces cell death in the absence of overexpressed epidermal growth factor receptor and ErbB2 in various human cancer cell lines. Cancer Invest. 2010; 28:505–14.

18. Kwok TT, Sutherland RM. Cell cycle dependence of epidermal growth factor induced radiosensitization. Int J Radiat Oncol Biol Phys. 1992; 22:525–7.

19. Kwon EK, Lee SH, Kim K, Wu HG, Lee SW. Differential effects of recombinant human EGF on proliferation and radiation survival of normal fibroblast and cancer cell lines. Open Transl Med J. 2009; 1:9–15.

20. Chiu B, Mirkin B, Madonna MB. Novel action of epidermal growth factor on caspase 3 and its potential as a chemotherapeutic adjunct for neuroblastoma. J Pediatr Surg. 2007; 42:1389–95.

21. Fan Z, Lu Y, Wu X, DeBlasio A, Koff A, Mendelsohn J. Prolonged induction of p21Cip1/WAF1/CDK2/PCNA complex by epidermal growth factor receptor activation mediates ligand-induced A431 cell growth inhibition. J Cell Biol. 1995; 131:235–42.

22. Jakus J, Yeudall WA. Growth inhibitory concentrations of EGF induce p21 (WAF1/Cip1) and alter cell cycle control in squamous carcinoma cells. Oncogene. 1996; 12:2369–76.
23. Bromberg JF, Fan Z, Brown C, Mendelsohn J, Darnell JE Jr. Epidermal growth factor-induced growth inhibition requires Stat1 activation. Cell Growth Differ. 1998; 9:505–12.
Fig. 1.
Treatment groups, dose and schedules in A431 xenograft models of nude mice. EGF, epidermal growth factor; fx, fractions. Group I, no treatment; group II, EGF for 6 days; group III, EGF for 20 days; group IV, radiotherapy; group V, radiotherapy plus concomitant EGF.
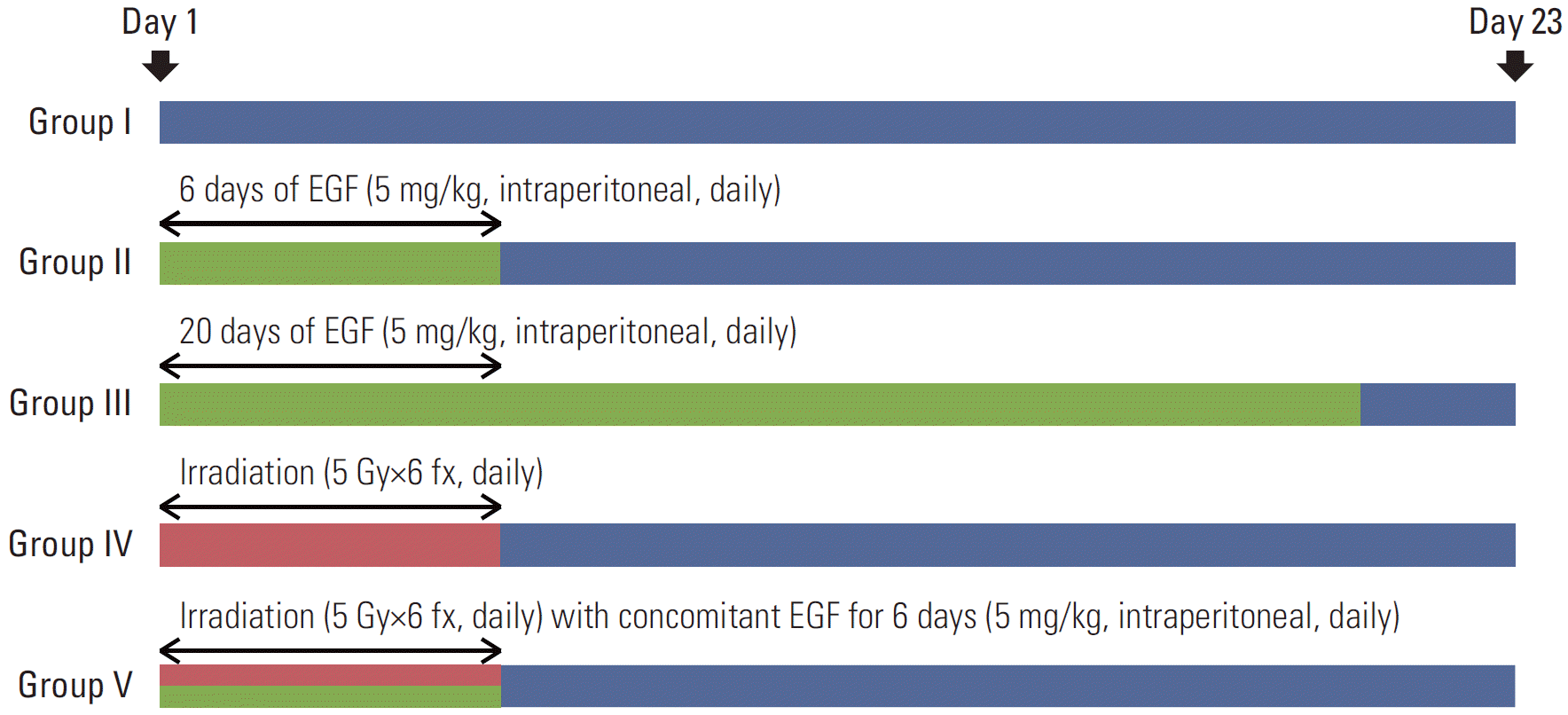
Fig. 2.
Changes in mean relative tumor volumes in group I (no treatment) versus II (epidermal growth factor [EGF] for 6 days) and IV (radiotherapy [RT], 30 Gy/6 fractions [fx]) versus V (RT [30 Gy/6 fx] with concomitant EGF for 6 days) after treatment. Error bars indicate the standard error of the mean relative tumor volumes for three independent experiments.
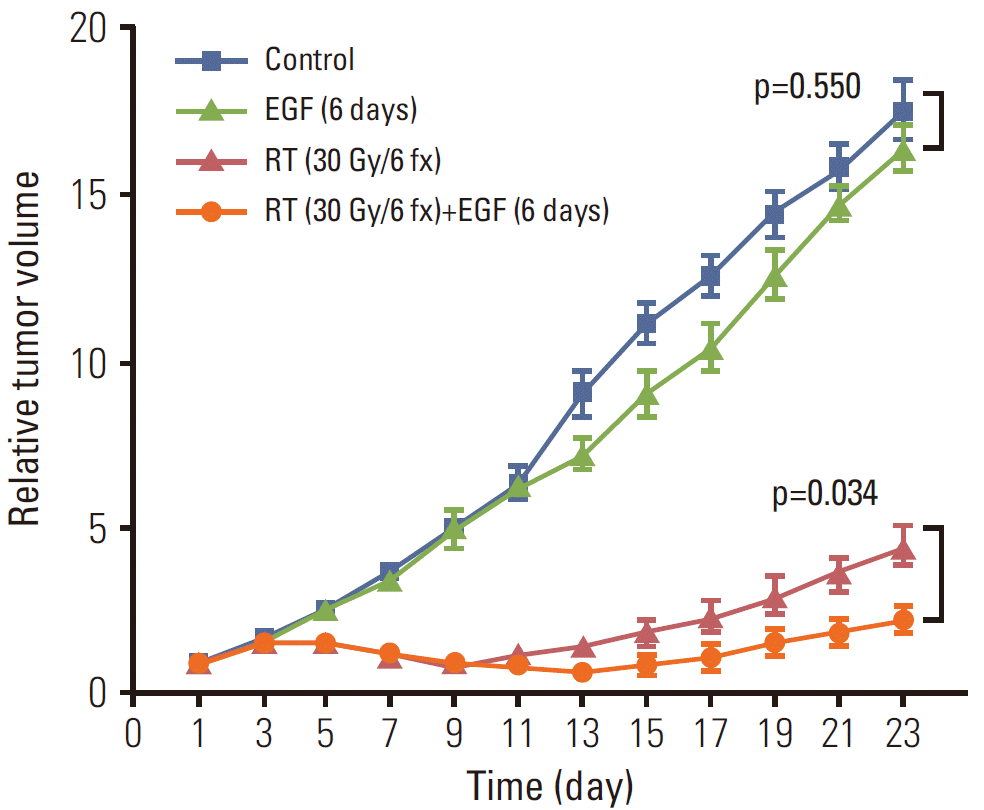
Fig. 3.
Changes in mean relative tumor volumes in group I (no treatment), II (epidermal growth factor [EGF] for 6 days), and III (EGF for 20 days) after treatment. Error bars indicate the standard error of the mean relative tumor volumes for three independent experiments.
Fig. 4.
Immunohistochemistry of anti-epidermal growth factor receptor (EGFR) antibody: paraffin-embedded tumor sections of the group I (no treatment) (A) and III (epidermal growth factor for 20 days) (B). A431 (human epidermoid carcinoma) tumor tissues from all five groups showed strong expression of EGFR (×200, day 0).
Fig. 5.
Immunohistochemistry of anti-cleaved caspase-3 antibody (paraffin-embedded tissue sections). Representative staining results of group IV (radiotherapy [RT], 30 Gy/6 fractions [fx]; day 12) (A, ×200; B, ×400) and group V (RT [30 Gy/6 fx] with concomitant epidermal growth factor for 6 days; day 12) (C, ×200; D, ×400). The arrowheads indicate positive cytoplasmic uptakes of active caspase-3.
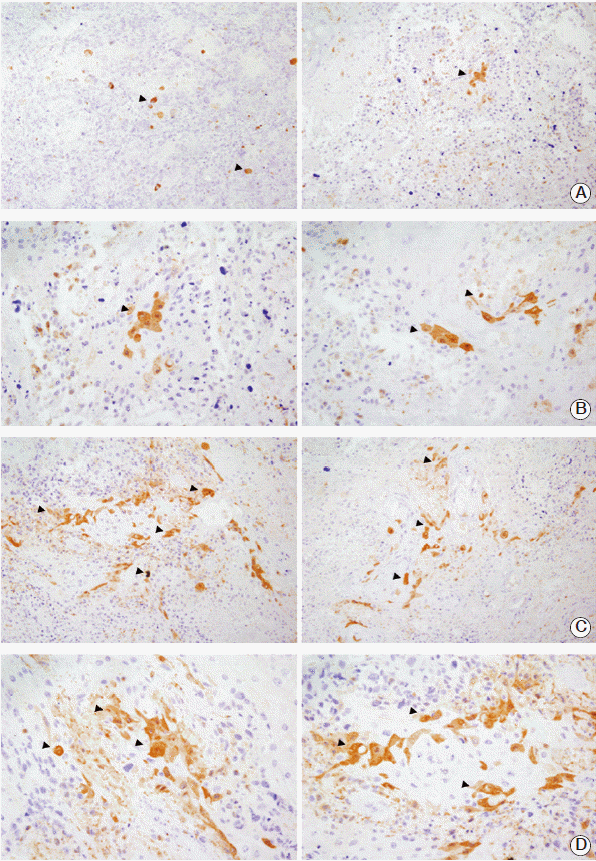
Fig. 6.
Hematoxylin and eosin staining of major normal organs. Microscopic findings of liver, lung, and kidney, respectively (×200). (A) Group I (no treatment; day 23). (B) Group III (epidermal growth factor [EGF] for 20 days; day 23). (C) Group V (radiotherapy, 30 Gy/6 fractions with concomitant EGF for 6 days; day 23). (D) Additional mice (EGF for 20 days without tumor inoculation-after 6 months of follow-up).
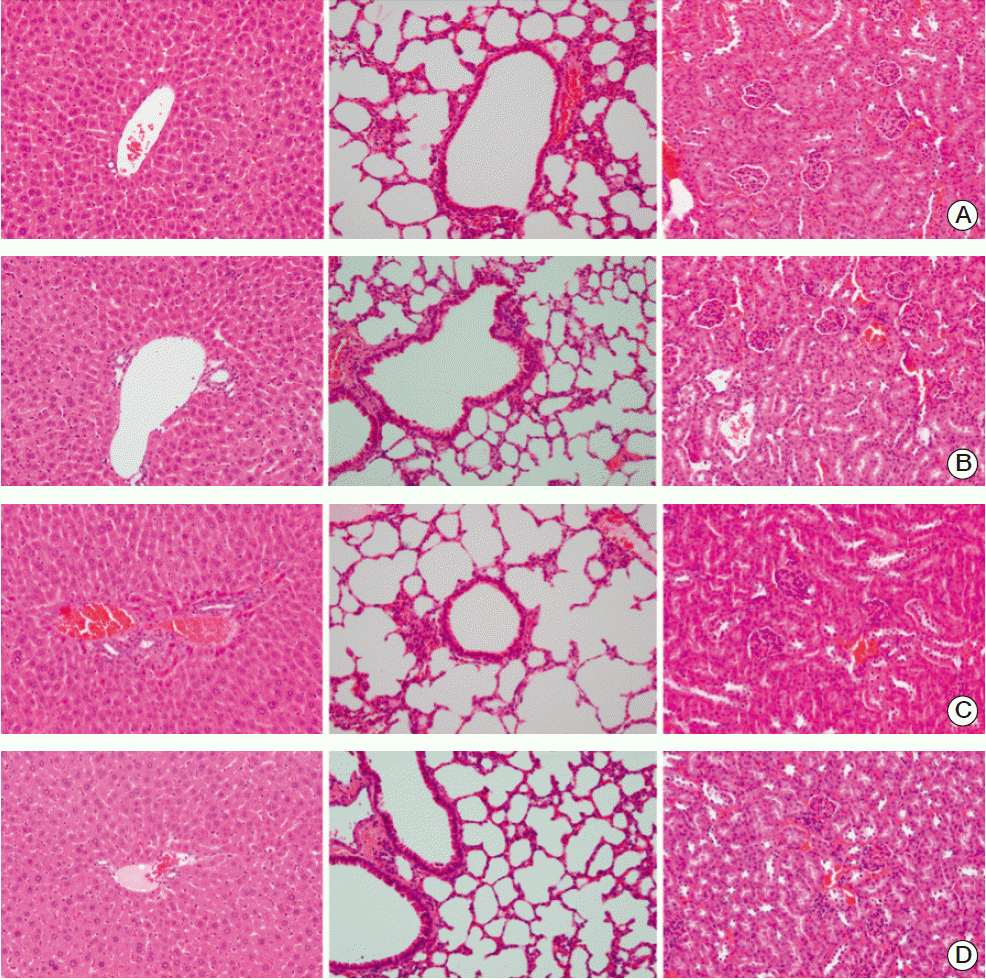
Table 1.
Scoring of EGFR immunohistochemistry
Reprinted from Licitra et al. [16] with permission of Oxford University Press. EGFR, epidermal growth factor receptor.
Table 2.
Immunohistochemistry of cleaved caspase-3 and EGFR of tumor tissues (day 12)
| Variable | I (control) | II (EGFx6) | III (EGFx20) | IV (RT) | V (RT+EGF) |
|---|---|---|---|---|---|
| EGFR | High | High | High | High | High |
| Cleaved caspase-3 Apoptosis (%)a) | 3 | 7 | 6 | 10 | 25 |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download