Abstract
Purpose
This study was conducted to evaluate the treatment outcomes following definitive bimodality concurrent chemoradiotherapy (CCRT) in patients with inoperable N2-positive stage IIIA (N2-IIIA) non-small cell lung cancer (NSCLC).
Materials and Methods
From May 1997 to December 2012, 65 out of 633 patients with N2-IIIA NSCLC received bimodality therapy. The treatment modality was selected during/after neoadjuvant CCRT in 21 patients or primarily at diagnosis in 44 through a multidisciplinary consensus meeting. The median age was 65 years (range, 36 to 76 years). Sixty patients (92.3%) had clinically evident N2 disease, while 22 (33.8%) had multi-station N2 involvement. The median radiation therapy dose was 66 Gy in 33 fractions, while the dose was elevated to 72 Gy in 13 patients who had a treatment break due to delayed decision regarding resectability. The most frequent chemotherapy regimen was weekly paclitaxel or docetaxel plus cisplatin or carboplatin (54, 83.1%).
Results
During the median follow-up of 18.8 months (range, 1.6 to 173.1 months), 34 patients (52.3%) experienced disease progression, with distant metastasis being the most common first treatment failure pattern (23, 34.8%). The median and 2-year rates of progression-free survival were 18.8 months and 45.9%, respectively. The median and 2-year rates of overall survival were 28.6 months and 50.1%, respectively.
The selection of an optimal treatment modality for patients with N2-positive stage IIIA (N2-IIIA) non-small cell lung cancer (NSCLC) has been a major controversial issue in thoracic oncology. A multimodality approach rather than a single modality therapy has been preferred [1], and the current National Comprehensive Cancer Network (NCCN) guideline recommends definitive concurrent chemoradiotherapy (CCRT) as the category 1 option, while induction chemotherapy, with or without radiation therapy (RT), and surgical resection is the alternative option [2]. The recent practice guidelines from the American College of Chest Physicians (ACCP) primarily recommends either definitive CCRT or induction therapy followed by surgery in the patients with discrete N2-IIIA NSCLC over either surgery or RT alone [3]. Both guidelines have multimodality treatment in common, and the ACCP guideline recommends that the treatment plan be made with input from a multidisciplinary team [3].
A multidisciplinary lung cancer team was established at Samsung Medical Center (SMC) when it was opened in 1994. Since then, SMC’s lung cancer team has actively implemented decision-making during lung cancer therapy, which has consisted of a full spectrum of lung cancer diagnoses and treatments including pulmonology, thoracic surgery, radiation oncology, medical oncology, diagnostic radiology, pathology, and nuclear medicine. Because of frequent locoregional failures following definitive CCRT without surgery [4,5], we have incorporated surgical resection following preoperative CCRT since 1997, and this trimodality therapy strategy has been the primary policy at SMC during treatment of patients with N2-IIIA NSCLC. Following trimodality therapy, the authors reported that the 5-year progression-free survival (PFS) and overall survival (OS) rates were 26.9% and 43.3%, respectively [6]. Although this strategy has demonstrated improved and favorable locoregional control and survival outcomes, surgical resection cannot be undertaken following preoperative CCRT in some patients. Accordingly, these patients underwent definitive CCRT without surgical resection (bimodality therapy). There are two main clinical settings in which bimodality therapy is undertaken at SMC. First, treatment policy changes from trimodality to bimodality therapy during or after the preoperative CCRT course, and second, bimodality therapy is primarily recommended from the onset of treatment. Typical patient factors necessitating a change in treatment policy include poor to marginal cardio-pulmonary function status, medical comorbidity, and the patient’s refusal of surgical resection. Typical disease factors leading to such a change include extensive and infiltrative nature of the tumor and/or lymph nodes that are judged to impede curative resection or necessitate pneumonectomy rather than lobectomy. The purpose of this study was to evaluate the treatment outcomes following bimodality therapy in patients with N2-IIIA NSCLC at SMC.
From May 1997 to December 2012, a total of 633 patients were diagnosed as having N2-IIIA NSCLC at SMC based on the American Joint Committee on Cancer (AJCC) seventh edition cancer staging manual (Fig. 1). After physiologic staging, 528 patients (83.4%) were judged to be medically operable with resectable or marginally resectable disease, while 105 (16.6%) were either medically inoperable or had unresectable disease because of the extensive and infiltrative nature of the tumor and/or lymph nodes. According to the SMC lung cancer center treatment policy, trimodality therapy including preoperative CCRT and surgical resection was used in 528 eligible patients. However, the planned preoperative CCRT course could not be completed in 51 patients (9.7%) due to disease progression in 22 (4.2%), patient refusal in 17 (3.2%), treatment-related mortality in six (1.1%) morbidity in four (0.8%), and other co-morbidity in two (0.4%). After re-assessment of resectability following preoperative CCRT, 456 patients (86.4%) underwent curative resection as initially planned, while the remaining 21 (4.0%), who had a presumed high risk of surgical resection or refused surgical resection, received boost CCRT (bimodality group I, hereafter). Among 105 patients who had unresectable disease at diagnosis, 61 who were not fit for aggressive treatment received either RT alone (n=50) or chemotherapy alone (n=11). Definitive CCRT was recommended to 44 patients as a primary therapy (bimodality group II, hereafter). After approval by the Institutional Review Board (2013-08-085), the medical and RT records of the 65 patients in bimodality groups I and II were retrospectively reviewed.
The median RT dose was 66 Gy in 33 fractions using 4-10 MV photon beams generated by a linear accelerator (Varian Medical Systems Inc., Palo Alto, CA). A median treatment break of 36 days was imposed during the RT course for 13 patients in bimodality group I, mainly because judgment on resectability was made 4 to 6 weeks after preoperative CCRT. To compensate for this unintended treatment break, the total RT dose was intentionally escalated to a median of 72 Gy in these patients. Therefore, the mean RT dose was higher in bimodality group I (p=0.002). The most common chemotherapeutic regimen during RT was weekly paclitaxel or docetaxel plus cisplatin or carboplatin in 54 patients (83.1%), followed by cisplatin plus oral etoposide at 4-week intervals in 11 patients (16.9%). Thirteen patients received 1 to 4 cycles of additional consolidation chemotherapy following bimodality therapy.
The primary endpoints of the current study were PFS and OS, and the secondary endpoints were in-field locoregional control (LRC) and patterns of disease progression. The durations of observation were calculated from the first date of CCRT until the date of event, death or censoring. Disease progression included any type of treatment failure, while in-field locoregional progression was defined as persistent or newly developing lesion within the actual RT target volume. Neither out-field remote intrathoracic failure nor hematogenous lung metastasis were considered as locoregional progression. t tests were used to compare continuous variables such as age, forced expiratory volume in 1 second, tumor size, and radiation dose. Chi-square or Fisher exact tests were used to compare categorical variables between treatment groups. The rates of PFS, OS, and in-field LRC were calculated using the Kaplan-Meier method and compared using the log-rank test. A p-value of ≤ 0.05 was considered statistically significant, and SAS software (ver. 9.1.3, SAS Institute Inc., Cary, NC) was used for all statistical analyses.
The patients’ characteristics are summarized in Table 1. The median age of the 65 patients in both groups was 65 years (range, 36 to 76 years), and the vast majority of the patients were male (59, 90.8%) and ex-smokers (60, 92.3%). Squamous cell carcinoma was the most common histology in 39 patients (60.0%), followed by adenocarcinoma in 22 (33.8%). Based on initial imaging studies, which included chest-computed tomography scan and/or positron emission tomography scan, the median tumor size was 4.3 cm (range, 1.0 to 11.6 cm), and cT1-2 stages were more common than cT3 (41 vs. 24). The majority of the patients (60, 92.3%) had clinically evident N2-positive disease, and about half (33, 50.8%) underwent histologic confirmation. Five patients (7.7%) with clinically N2-negative disease were confirmed to have N2 disease based on histological analysis. The procedures for histologic confirmation of N2 disease were endobronchial ultrasound-guided transbronchial needle aspiration in 17 patients, mediastinoscopic biopsy in 11, videoassisted thoracoscopic biopsy in two, endoscopic transesophageal ultrasound guided biopsy in one, bronchoscopic biopsy in one, and open excision of the lymph node in one. Based on all clinical evaluation tools, 43 and 22 patients (66.2% and 33.8%, respectively) were known to have single and multi-station N2 involvement, respectively.
During the median follow-up duration of 18.8 months (range, 1.6 to 173.1 months), 34 patients (52.3%) showed disease progression (Table 2). The most common first treatment failure was distant metastasis, which was observed in 23 patients (34.8%), followed by locoregional progression in 16 (24.6%). Additionally, 12 out of 16 patients with locoregional progression had failure within the RT volume. The most frequent distant metastatic organs were the brain in nine patients, followed by the adrenal gland in four. The median PFS was 18.8 months, and the 2- and 5-year PFS rates were 45.9% and 30.5%, respectively (Fig. 2A). In-field LRC rates at 2 and 5 years were 74.3% and 56.3%, respectively. A total of 39 patients (60.0%) died during the follow-up period. Pulmonary causes such as pneumonia, pneumonitis, and acute respiratory distress syndrome were responsible for seven deaths, which occurred at a median of 4.0 months (range, 2.3 to 8.5 months). There were three intercurrent deaths from hepatocellular carcinoma, myocardial infarction, and gastrointestinal bleeding. The median OS was 28.6 months, and the 2- and 5-year OS rates were 50.1% and 33.3%, respectively (Fig. 2B). Univariate analysis revealed that the cisplatin plus oral etoposide regimen was associated with significantly better PFS and OS (Table 3), and females tended to have a better OS. There were no significant differences in survival outcomes between bimodality groups I and II. Other variables such as cT stage, involvement of multistation lymph nodes, and treatment break did not affect survival outcomes.
N2-IIIA NSCLC involves potential heterogeneity in mediastinal lymph node involvement, which could influence the decision regarding the treatment plan among various options. A survey study showed that choice of treatment plan differed according to the extent of mediastinal involvement [7]. In the case of single-node disease, 92% of the oncologists incorporated surgical resection into a treatment plan, while definitive chemoradiation was mostly preferred in the case of bulky multi-station N2 disease. Interestingly, 48% of the treatment plans still included surgery in the second scenario. In addition to the heterogeneity of N2 disease, there might also be heterogeneity in the physician’s treatment plan preference. Among patients who received trimodality therapy for N2-IIIA NSCLC at our institution, 66.8% and 36.6% had clinically evident and multi-station N2 disease, respectively [6].
Our institution has adopted a multidisciplinary approach to management of patients with lung cancer, and tri- or bi-modality treatment showed consistently favorable outcomes. Although not all patients had histological confirmation of involvement of the N2 lymph node in this study, most were found to have clinically evident N2 disease upon imaging analyses, and about one-third of the patients had multi-station N2 disease. Resectability was assessed before and/or during the course of CCRT, in accordance with the multidisciplinary team approach described above. Our rationale for increasing radiation dose to compensate for the unintended treatment break applied in 13 patients in bimodality group I is very difficult to validate based on the current study results. Twenty-one patients in bimodality group I, 13 of whom had treatment break and increased radiation dose, showed similar clinical outcomes to those in bimodality group II (Table 3). Naturally, it is desirable to avoid or shorten the treatment break whenever possible; however, when such a break cannot be avoided, an effort to intensify the boost CCRT seems a reasonable approach.
Trimodality therapy that includes surgical resection following preoperative CCRT in physiologically fit patients with N2-IIIA NSCLC has long been the primary recommendation at SMC, and subsequent clinical outcomes have recently been reported [6]. Definitive bimodality therapy has been recommended to physiologically fit patients who are poor candidates for surgical resection due to either increased surgical risk or patient refusal of trimodality therapy. Direct comparisons of the outcomes following tri- and bimodality therapy might be biased owing to selection bias and heterogeneity in the patient characteristics in this retrospective study. A randomized controlled phase III trial compared the two treatment modalities and determined that the median OS was 23.6 months in patients who underwent surgical resection and 22.2 months in patients who underwent continued radiotherapy (p=0.24), which was similar to the results of the present study [8]. The corresponding PFS were 12.8 and 10.5 months, respectively, in the two treatment groups (p=0.017). Although no survival difference was observed between the two treatment modalities, a survival benefit was observed in patients who received lobectomy after neoadjuvant CCRT (median OS, 33.6 months vs. 21.7 months; p=0.002). Decreased survival in pneumonectomized patients was suggested as the main reason for the limited survival benefit following trimodality treatment. Improved survival after CCRT plus lobectomy and decreased survival after CCRT plus pneumonectomy was also observed in recent studies from our institution and the National Cancer Database [6,9]. Negative survival influence following pneumonectomy has clinical implications in terms of treatment selection in patients with N2-IIIA NSCLC. Pneumonectomy candidates appear to be more suitable for definitive bimodality therapy than trimodality therapy, while candidates who do not require pneumonectomy might benefit from trimodality therapy.
Survival differences between chemotherapy regimens should be interpreted with caution. Treatment results after CCRT with cisplatin plus oral etoposide followed by consolidation chemotherapy were previously reported [10], and the median PFS and 2-year PFS rate were 12.3 months and 40%, respectively. Because more than half of the patients in that study had N3 disease, only a small portion of patients with favorable prognosis should have been included. In addition, the role of the docetaxel-based combination regimen has been widely examined and its effectiveness demonstrated [11-15]. Thus, valid comparison between chemotherapy regimens is not feasible in the current retrospective study. Rather, the role of consolidation chemotherapy should be investigated in the future [15,16]. Because consolidation chemotherapy was rarely applied in the current study, it is still not clear whether further improvement of treatment outcomes is possible from consolidation chemotherapy. A multinational phase III randomized trial that compares CCRT with or without consolidation chemotherapy in inoperable stage III NSCLC was initiated by the authors at SMC [17]. The interim analysis showed a median OS of 20.7 and 21.1 months in the CCRT alone arm and consolidation arm, respectively (p=0.49). In addition, the corresponding median time to progression was 9.0 and 13.9 months, respectively (p=0.19). Long-term results are expected to validate the feasibility and efficacy of consolidation chemotherapy.
It should be noted that the current study has some potential limitations as a result of its retrospective nature, as well as the small number of patients and heterogeneous patient population. As recommended by the current NCCN guideline, definitive bimodality therapy could be applied as a primary treatment in patients with N2-IIIA NSCLC that is unresectable or necessitates pneumonectomy, while trimodality could be considered for candidates that do not need pneumonectomy. Additional efforts to avoid and minimize treatment break should be applied through a multidisciplinary team approach.
References
1. Martini N, Kris MG, Ginsberg RJ. The role of multimodality therapy in locoregional non-small cell lung cancer. Surg Oncol Clin N Am. 1997; 6:769–91.

2. National Comprehensive Cancer Network. NCCN guidelines version 3, 2014. Non-small cell lung cancer [Internet] . Fort Washington, PA: National Comprehensive Cancer Network;[cited 2014 Jun 9] . Available from: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
3. Ramnath N, Dilling TJ, Harris LJ, Kim AW, Michaud GC, Balekian AA, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013; 143(5 Suppl):e314S.
4. Fournel P, Robinet G, Thomas P, Souquet PJ, Lena H, Vergnenegre A, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 Study. J Clin Oncol. 2005; 23:5910–7.
5. Curran WJ Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011; 103:1452–60.

6. Lee H, Ahn YC, Pyo H, Kim B, Oh D, Nam H, et al. Pretreatment clinical mediastinal nodal bulk and extent do not influence survival in N2-positive stage IIIA non-small cell lung cancer patients treated with trimodality therapy. Ann Surg Oncol. 2014; 21:2083–90.

7. Tanner NT, Gomez M, Rainwater C, Nietert PJ, Simon GR, Green MR, et al. Physician preferences for management of patients with stage IIIA NSCLC: impact of bulk of nodal disease on therapy selection. J Thorac Oncol. 2012; 7:365–9.

8. Albain KS, Swann RS, Rusch VW, Turrisi AT 3rd, Shepherd FA, Smith C, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009; 374:379–86.

9. Koshy M, Fedewa SA, Malik R, Ferguson MK, Vigneswaran WT, Feldman L, et al. Improved survival associated with neoadjuvant chemoradiation in patients with clinical stage IIIA(N2) non-small-cell lung cancer. J Thorac Oncol. 2013; 8:915–22.

10. Park J, Ahn YC, Kim H, Lee SH, Park SH, Lee KE, et al. A phase II trial of concurrent chemoradiation therapy followed by consolidation chemotherapy with oral etoposide and cisplatin for locally advanced inoperable non-small cell lung cancers. Lung Cancer. 2003; 42:227–35.

11. Scagliotti GV, Turrisi AT 3rd. Docetaxel-based combinedmodality chemoradiotherapy for locally advanced non-small cell lung cancer. Oncologist. 2003; 8:361–74.

12. Pillot G, Govindan R. The role of docetaxel in N2 locally advanced non-small-cell lung cancer. Clin Lung Cancer. 2005; 7 Suppl 3:S87–92.

13. Saloustros E, Georgoulias V. Docetaxel in the treatment of advanced non-small-cell lung cancer. Expert Rev Anticancer Ther. 2008; 8:1207–22.

14. Oh IJ, Kim KS, Kim YC, Ban HJ, Kwon YS, Kim YI, et al. A phase III concurrent chemoradiotherapy trial with cisplatin and paclitaxel or docetaxel or gemcitabine in unresectable non-small cell lung cancer: KASLC 0401. Cancer Chemother Pharmacol. 2013; 72:1247–54.

15. Saitoh J, Saito Y, Kazumoto T, Kudo S, Yoshida D, Ichikawa A, et al. Concurrent chemoradiotherapy followed by consolidation chemotherapy with bi-weekly docetaxel and carboplatin for stage III unresectable, non-small-cell lung cancer: clinical application of a protocol used in a previous phase II study. Int J Radiat Oncol Biol Phys. 2012; 82:1791–6.

16. Eroglu C, Orhan O, Unal D, Dogu GG, Karaca H, Dikilitas M, et al. Concomitant chemoradiotherapy with docetaxel and c isplatin followed by consolidation chemotherapy in locally advanced unresectable non-small cell lung cancer. Ann Thorac Med. 2013; 8:109–15.
17. Park K, Ahn Y, Chen M, Cho E, Kim J, Min Y, et al. A multinational phase III randomized trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non-small cell lung cancer (CCheIN): interim analysis. J Clin Oncol. 2009; 27(15S):7538.

Fig. 1.
Treatment flow diagram for patients with N2-positive stage IIIA (N2-IIIA) non-small cell lung cancer (NSCLC).
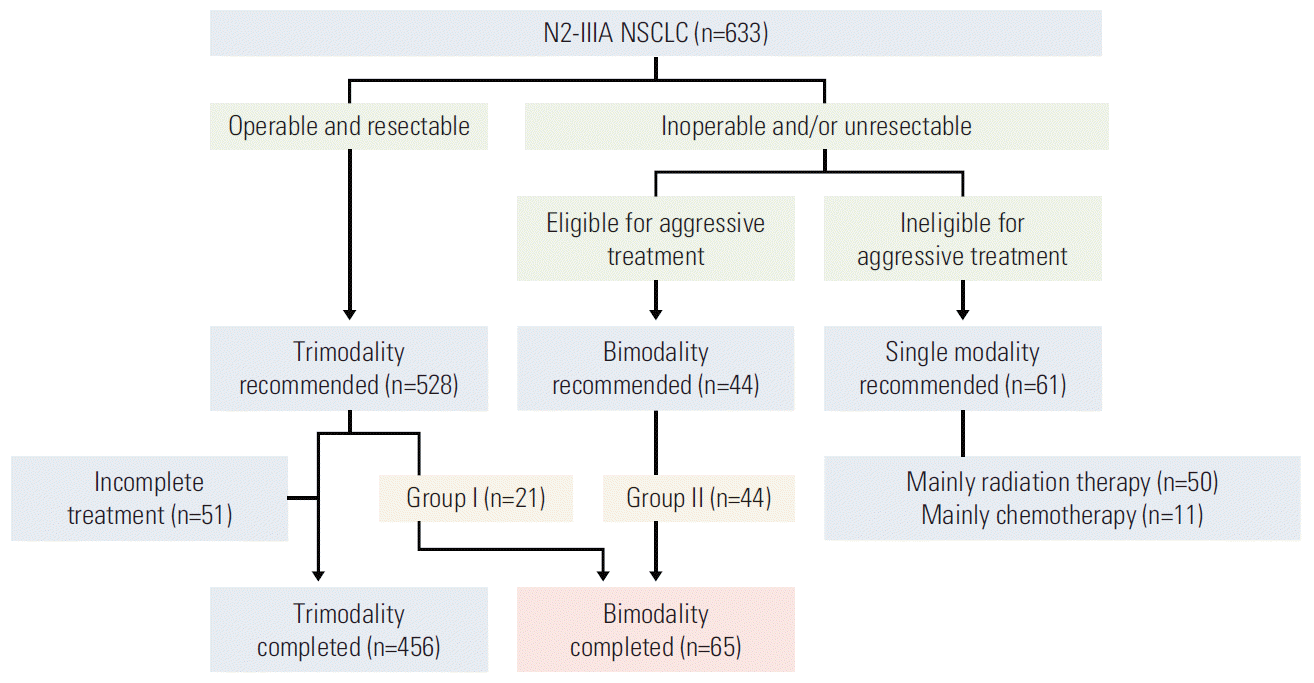
Fig. 2.
Progression-free survival (A) and overall survival (B) after definitive bimodality concurrent chemoradiotherapy in patients with N2-positive stage IIIA non-small cell lung cancer.
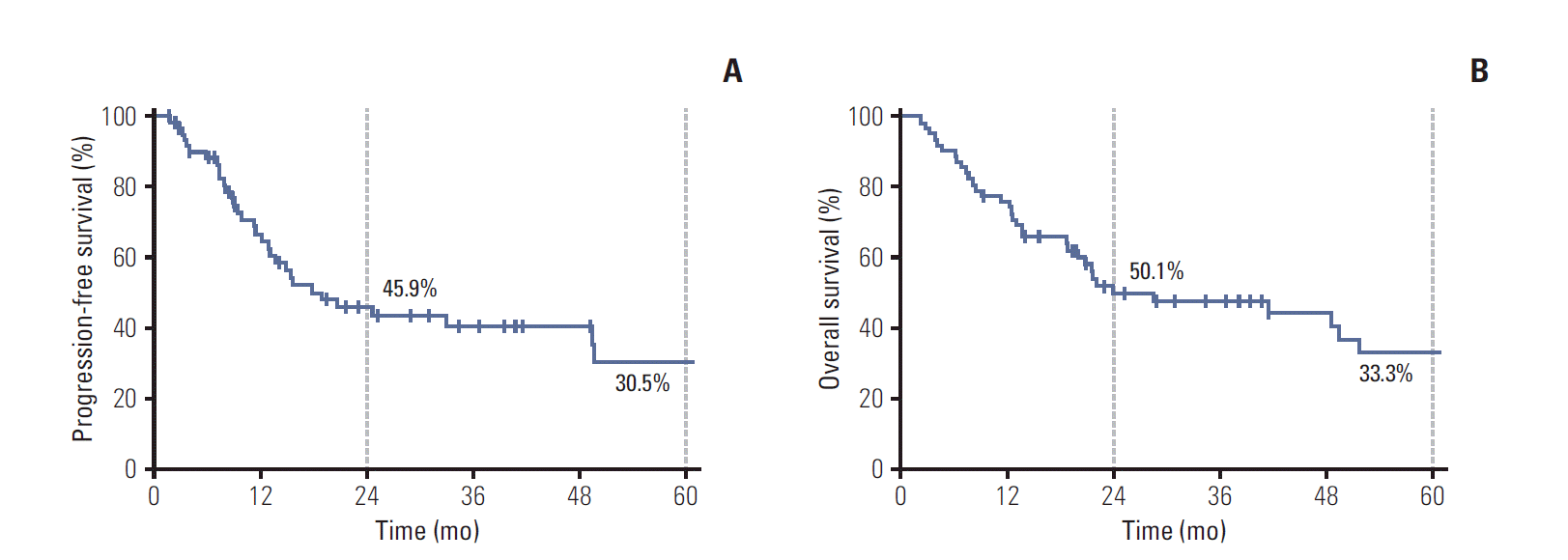
Table 1.
Clinical characteristics
| Characteristic | Total (n=65) | Group I (n=21) | Group II (n=44) | p-value |
|---|---|---|---|---|
| Age (yr) | 65 (36-76) | 65 (47-76) | 63 (36-75) | 0.365 |
| Gender | 1.000a) | |||
| Male | 59 (90.8) | 19 (90.5) | 40 (90.9) | |
| Female | 6 (9.2) | 2 (9.5) | 4 (9.1) | |
| Smoking history | 0.655a) | |||
| Yes | 60 (92.3) | 19 (90.5) | 41 (93.2) | |
| No | 5 (7.7) | 2 (9.5) | 3 (6.8) | |
| FEV1 (L) | 2.44 (0.92-4.26) | 2.41 (1.24-3.35) | 2.47 (0.92-4.26) | 0.411 |
| Histology | 0.942 | |||
| Squamous cell carcinoma | 39 (60.0) | 13 (61.9) | 26 (59.1) | |
| Adenocarcinoma | 22 (33.8) | 7 (33.3) | 15 (34.1) | |
| Others | 4 (6.2) | 1 (4.8) | 3 (6.8) | |
| Tumor size (cm) | 4.3 (1.0-11.6) | 3.7 (2.0-9.1) | 5.8 (1.0-11.6) | 0.105 |
| Clinical T classification | 0.020 | |||
| cT1-2 | 41 (63.1) | 9 (42.9) | 32 (72.7) | |
| cT3 | 24 (36.9) | 12 (57.1) | 12 (27.3) | |
| Extent of N2 disease | 0.052 | |||
| Clinically evident-histologic confirmation | 32 (49.2) | 10 (47.6) | 22 (50.0) | |
| Clinically evident+histologic confirmation | 28 (43.1) | 7 (33.3) | 21 (47.7) | |
| Clinically silent but histologically confirmed | 5 (7.7) | 4 (19.0) | 1 (2.3) | |
| Level of N2 disease | 0.952 | |||
| Single station | 43 (66.2) | 14 (66.7) | 29 (65.9) | |
| Multi-station | 22 (33.8) | 7 (33.3) | 15 (34.1) |
Table 2.
Patterns of first treatment failure
Table 3.
Prognostic factors affecting survival outcomes upon univariate analysis




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download