Abstract
Purpose
This retrospective cohort study was conducted to estimate the optimal interval for gastric cancer screening in Korean adults with initial negative screening results.
Materials and Methods
This study consisted of voluntary Korean screenees aged 40 to 69 years who underwent subsequent screening gastroscopies after testing negative in the baseline screening performed between January 2007 and December 2011. A new case was defined as the presence of gastric cancer cells in biopsy specimens obtained upon gastroscopy. The follow-up periods were calculated during the months between the date of baseline screening gastroscopy and positive findings upon subsequent screenings, stratified by sex and age group. The mean sojourn time (MST) for determining the screening interval was estimated using the prevalence/incidence ratio.
Results
Of the 293,520 voluntary screenees for the gastric cancer screening program, 91,850 (31.29%) underwent subsequent screening gastroscopies between January 2007 and December 2011. The MSTs in men and women were 21.67 months (95% confidence intervals [CI], 17.64 to 26.88 months) and 15.14 months (95% CI, 9.44 to 25.85 months), respectively.
Gastric cancer is the second leading type of cancer occurrence in Korea [1]. The mortality related to gastric cancer is decreasing because of the dissemination of gastric cancer screening programs as well as advances in surgical techniques, chemotherapy, and radiotherapy [2]. There is a national mass screening program for gastric cancer in Korea, which recommends biennial screening with gastroscopy or upper gastrointestinal series (UGIS) in adults older than 40 years of age [3].
This screening guideline for gastric cancer in asymptomatic normal-risk adults consists of age at onset, modality, and intervals. Gastroscopy as the primary test for early gastric cancer (EGC) screening would be recommended [4]. However, the optimal interval has been the subject of debate [5]. Although some studies have evaluated the effectiveness of biennial gastric cancer screening by gastroscopy [6,7], others have suggested screening intervals of 1 to 1.5 or 3 years to detect EGC by gastroscopy or UGIS [8-11]. However, the outcomes of screening by intervals were not analyzed separately [7,12]. These studies were also conducted using a case series design [6,7,9,10,12]. Accordingly, optimal screening intervals for EGC detection should be determined through well designed studies [7]. Recently, a report to estimate the mean sojourn time (MST) for gastric cancer screening in normal-risk Korean men was reported [13], and the authors suggested a further study for investigation of the MST in Korean women. Thus, this retrospective cohort study was conducted to estimate the optimal interval for gastric cancer screening in normal-risk Korean women and men.
The source population is the same as that in a previous study conducted by Bae et al. [13,14]. Gastroscopy is the primary modality for the initial and follow-up screening of gastric cancer. The inclusion and exclusion criteria for defining study participants were also the same as those used in the aforementioned study.
A new case was one in which gastric cancer cells were confirmed in biopsy specimens obtained upon gastroscopy, which were evaluated by gastrointestinal pathologists [15]. All screenees consented to gastroscopy and the use of personal data for research. This study protocol was approved by the Institutional Review Board of Jeju National University Hospital (File No. 2013-05-023) and was registered in the Clinical Research Information Service system, Korea Centers for Disease Control and Prevention (No. KCT0000772).
The variables and statistical methods were conducted as previously described [13]. The detection rate (R) per 100,000 screenees at the first (baseline) screening was calculated by dividing the number of biopsy-proven cases (D) by the total number of screenees. Based on the follow-up information, the incidence rate (I) per 100,000 screenee-months was calculated by dividing the number of new cases (N) by the total number of months of follow-up periods. The 95% confidence intervals (CIs) for incidence rates are based on the Poisson distribution.
As the sojourn time, defined as the duration of the preclinical detectable phase, is an unobserved quantity, the MST for determining the screening interval was estimated using the prevalence/incidence ratio [16,17]. This method of estimation is adequate when the incidence can be treated as a constant and the sojourn times are relatively short, such as in breast cancer, which has an estimated MST of 1-2 years [16,18]. The estimation model suggested that MST is calculated by dividing the prevalence of preclinical cancer (P) by the incidence (I). Prevalence is estimated by dividing the detection rate at first screening (R) by the sensitivity of the screening test (S). Because the sensitivity of a test is defined as the proportion of patients with positive findings, S could be estimated by dividing the detected cases (D) by the sum of D and the number of newly found cases (N) during the follow-up periods. However, a previous population-based study of gastric screening in normal-risk Koreans [10] showed that 48.9% of screened patients with gastric cancer had undergone a previous gastroscopy at least once; hence, N may be replaced with 2.05N. Thus MST=R/[I(D+2.05N)]. The upper and lower boundaries of MST were estimated based on the 95% CI of the incidence rates. Considering the cost-effectiveness and compliance of screening, the upper limit of MST was defined as an optimal interval [19]. All statistical analyses were performed using STATA ver. 12 (StataCorp, College Station, TX).
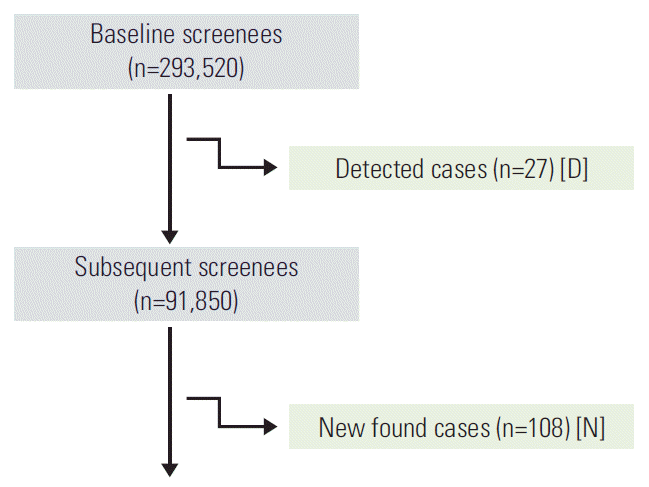
Fig. 1 shows the overall process of cohort construction and follow-up. A total of 293,520 screenees voluntarily participated in the Korea Medical Institute screening program for gastric cancer between January 2007 and December 2011. In the first screening (D), 27 cases were detected, giving a detection rate (R) of 9.20 per 100,000 screenees. Table 1 shows the distribution of detection rates by age and sex.
Among the subjects showing negative gastroscopy upon baseline gastric cancer screening, 91,850 (31.29%) underwent subsequent screening gastroscopies. A total of 108 incident cases (N) were found, with an incidence rate (I) of 4.51 per 100,000 screenee-months (95% CI, 3.69 to 5.44). Table 2 shows the distribution of incidence rates, follow-up periods (months), and the number of incidents by sex and age group.
Table 3 shows the estimated MSTs. The parameters were obtained from D and R in Table 1, and from I and N in Table 2. Although no cases of gastric cancer were detected upon baseline screening in women aged 60-69 years, the MSTs in men and women were 21.67 months (95% CI, 17.64 to 26.88 months) and 15.14 months (95% CI, 9.44 to 25.85 months), respectively.
While the upper boundaries of the MST in both men and women were about 24 months, the MST in all subjects aged 40-69 years was 18.81 months (95% CI, 15.56 to 22.93 months). These findings were in accordance with the results of a study conducted by Bae et al. [13]. However, the MST findings should be interpreted with caution because of the possibility of heterogeneity in MSTs between men and women. Specifically, the rarer detected (D) and newly found (N) cases in women resulted in a broader 95% CI than that noted for men. In addition, the MST in women aged 60-69 years could not be calculated because no cases were detected upon baseline screening.
The optimal interval for the early detection of cancer is determined by three major factors: doubling time [20], different survival times with screening [7,9], and detection rate of interval cancers [8,10,21]. Among these, the detection of interval cancers should depend on screening intervals and the validity of the screening test [22]. Considering these factors, the optimal interval was determined by obtaining the MST from the continuous-time Markov model [18].
To generate evidence for the detection of interval cancers, a follow-up cohort study and randomized controlled trial should be conducted. However, an intervention trial for healthy adults could not be performed due to the related ethical issues. To conduct an observational cohort study, subjects should undergo a subsequent screening test at the same organization and fill out a questionnaire regarding cancer history. The subjects were selected from the voluntary attendees of the major cancer screening programs provided by the Korea Medical Institute (KMI).
South Korea and Japan are the only countries that have established nationwide programs for stomach cancer screening to date [23]. The cost-utility of screening tools and the compliance of screenees, as well as MST should be considered when deciding screening interval. Based on these factors, the authors selected an optimal interval using the upper limit of 95% CI of MST.
Even though this was a retrospective cohort study, it is important to note that there were several limitations. First, approximately one of three voluntary screenees with negative results at the baseline gastric cancer screening underwent subsequent screening endoscopies during the study period (35.9% of the men and 24.8% of the women). Because there is a lower incidence of gastric cancer in women [1] and the number of detected (D) and newly found (N) cases in women was relatively small (with wider CIs), a follow-up study in women is required. Second, KMI supplies the screening service, but does not treat patients with the detected cancers. New cases of gastric cancer detected based on histologically positive findings in tissue biopsies obtained upon gastroscopy should be transferred to nearby cancer centers. Because the KMI database did not obtain information regarding cancer stage, the authors could not evaluate the optimal intervals by comparing early and advanced gastric cancer cases [6,7,9]. Finally, participation in subsequent screening could not be followed up. To calculate the exact sensitivity of gastroscopy used in the population-based screening, data for the subjects should be merged into the nationwide cancer registry database [1]. However, the authors could not attempt this because it is prohibited. To overcome this barrier, the authors extracted related information from a previous population-based study of gastric screening in average-risk Korean adults [10].
In conclusion, this study suggests that the optimal interval of subsequent gastric screening in both men and women is 24 months. The study provides further support for the 2-year interval recommended by the nationwide gastric cancer screening program. Further follow-up studies in women and investigation of the sensitivity of screening gastroscopy are required.
ACKNOWLEDGMENTS
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (No. 1320300). The authors thank Professor Chung Mo Nam, Department of Preventive Medicine, Yonsei University College of Medicine, Seoul, Korea, for advice regarding the bio-statistics.
References
1. Korea Central Cancer Registry. Annual report of cancer statistics in Korea in 2010 [Internet]. Goyang: National Cancer Center;2013. [cited 2014 Mar 13]. Available from: http://ncc.re.kr/manage/manage03_033_view.jsp?bbsnum=250&hSelSearch=&hTxtKeyword=¤t_page=1&cd=null.
2. Hosokawa O, Miyanaga T, Kaizaki Y, Hattori M, Dohden K, Ohta K, et al. Decreased death from gastric cancer by endoscopic screening: association with a population-based cancer registry. Scand J Gastroenterol. 2008; 43:1112–5.

3. National Cancer Control Institute. National Cancer Screening
Program [Internet]. Goyang: National Cancer Center;[cited 2014 Mar 13]. Available from: http://ncc.re.kr/english/programs/progarms03.jsp.
4. Choi KS, Jun JK, Park EC, Park S, Jung KW, Han MA, et al. Performance of different gastric cancer screening methods in Korea: a population-based study. PLoS One. 2012; 7:e50041.

5. Riecken B, Pfeiffer R, Ma JL, Jin ML, Li JY, Liu WD, et al. No impact of repeated endoscopic screens on gastric cancer mortality in a prospectively followed Chinese population at high risk. Prev Med. 2002; 34:22–8.

6. Mori Y, Arita T, Shimoda K, Yasuda K, Yoshida T, Kitano S. Effect of periodic endoscopy for gastric cancer on early detection and improvement of survival. Gastric Cancer. 2001; 4:132–6.

7. Nam SY, Choi IJ, Park KW, Kim CG, Lee JY, Kook MC, et al. Effect of repeated endoscopic screening on the incidence and treatment of gastric cancer in health screenees. Eur J Gastroenterol Hepatol. 2009; 21:855–60.

8. Yoon H, Kim N, Lee HS, Shin CM, Park YS, Lee DH, et al. Effect of endoscopic screening at 1-year intervals on the clinicopathologic characteristics and treatment of gastric cancer in South Korea. J Gastroenterol Hepatol. 2012; 27:928–34.

9. Shiratori Y, Nakagawa S, Kikuchi A, Ishii M, Ueno M, Miyashita T, et al. Significance of a gastric mass screening survey. Am J Gastroenterol. 1985; 80:831–4.
10. Nam JH, Choi IJ, Cho SJ, Kim CG, Jun JK, Choi KS, et al. Association of the interval between endoscopies with gastric cancer stage at diagnosis in a region of high prevalence. Cancer. 2012; 118:4953–60.

11. Lee H, Min BH, Lee JH, Son HJ, Kim JJ, Rhee JC, et al. Survival outcome associated with the screening interval for gastric cancer in Korea. Digestion. 2011; 84:142–8.

12. Aida K, Yoshikawa H, Mochizuki C, Mori A, Muto S, Fukuda T, et al. Clinicopathological features of gastric cancer detected by endoscopy as part of annual health checkup. J Gastroenterol Hepatol. 2008; 23:632–7.

13. Bae JM, Shin SY, Kim EH. Mean sojourn time of preclinical gastric cancer in Korean men: a retrospective observational study. J Prev Med Public Health. 2014; 47:201–5.

14. Korea Medical Institute. Nationwide network [Internet]. Seoul: Korea Medical Institute;[cited 2014 Feb 10]. Available from: http://www.kmi.or.kr/kor/center/network/kmi.web.
15. American Cancer Society. Diagnosis of stomach cancer: biopsy [Internet]. Atlanta, GA: American Cancer Society;c2014. [cited 2014 Mar 13]. Available from: http://www.cancer.org/cancer/stomachcancer/detailedguide/stomach-cancer-diagnosis.
16. Draisma G, van Rosmalen J. A note on the catch-up time method for estimating lead or sojourn time in prostate cancer screening. Stat Med. 2013; 32:3332–41.

17. Pashayan N, Duffy SW, Pharoah P, Greenberg D, Donovan J, Martin RM, et al. Mean sojourn time, overdiagnosis, and reduction in advanced stage prostate cancer due to screening with PSA: implications of sojourn time on screening. Br J Cancer. 2009; 100:1198–204.

18. Day NE, Walter SD. Simplified models of screening for chronic disease: estimation procedures from mass screening programmes. Biometrics. 1984; 40:1–14.

19. Bae JM. Methodological issues for determining intervals of subsequent cancer screening. Epidemiol Health. 2014; 36:e2014010.

20. Tabar L, Fagerberg G, Day NE, Duffy SW, Kitchin RM. Breast cancer treatment and natural history: new insights from results of screening. Lancet. 1992; 339:412–4.
Table 1.
Baseline characteristics of the source population (n=293,520) according to gender and age groups
Table 2.
Baseline characteristics of study participants (n=91,850) that underwent subsequent screening gastroscopies after negative gastroscopy findings on the first screening stratified by gender and age group
| Gender | Age (yr) | No. (%) |
Subsequent screening endoscopy |
|||||
|---|---|---|---|---|---|---|---|---|
|
Follow-up |
New case | Incidence ratec) | 95% Confidence interval | |||||
| Totala) | Meanb) | Medianb) | ||||||
| Man | Total | 61,688 (100) | 1,633,216.49 | 26.48 | 24.21 | 91 | 5.57 | 4.49-6.84 |
| 40-49 | 42,498 (69.89) | 1,146,072.15 | 26.97 | 24.41 | 52 | 4.54 | 3.39-5.96 | |
| 50-59 | 15,801 (26.61) | 402,611.19 | 25.48 | 23.92 | 30 | 7.45 | 5.03-10.6 | |
| 60-69 | 3,389 (5.49) | 84,533.16 | 24.94 | 23.85 | 9 | 10.65 | 4.87-20.2 | |
| Woman | Total | 30,162 (100.0) | 764,092.19 | 25.33 | 23.92 | 17 | 2.22 | 1.30-3.56 |
| 40-49 | 18,770 (62.23) | 484,270.88 | 25.80 | 24.02 | 9 | 1.86 | 0.85-3.53 | |
| 50-59 | 8,622 (28.59) | 212,063.38 | 24.60 | 23.69 | 6 | 2.83 | 1.04-6.16 | |
| 60-69 | 2,770 (9.18) | 67,757.93 | 24.46 | 23.69 | 2 | 2.95 | 0.36-10.7 | |
| All | Total | 91,850 (100) | 2,397,308.68 | 26.10 | 24.11 | 108 | 4.51 | 3.69-5.44 |
Table 3.
Estimation of the mean sojourn time (MST) by gender and age group
D and R refer to the number of cases detected and the rate of detection at the first screening, respectively, as presented in Table 1. N and I refer to the number of newly identified cases and the incidence rate, respectively, as presented in Table 2. S refers to the sensitivity, D/(D+2.05N). MST refers to the mean sojourn time, R/(IS); MST_L and MST_U refer to the lower and upper boundaries of MST based on the 95% confidence intervals of incidence rates (I).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download