Abstract
Purpose
Bone marrow biopsy is a standard method for the evaluation of bone marrow infiltration by lymphoma; however, it is an invasive and painful procedure. Fluorodeoxyglucose positron emission tomography–computed tomography (FDG PET-CT) is a noninvasive imaging technique with the potential to detect bone marrow involvement by lymphoma.
Materials and Methods
We retrospectively reviewed medical records of lymphoma patients. All patients were examined by FDG PET-CT and iliac crest bone marrow biopsy for initial staging work-up.
Results
The study population comprised 94 patients (median age, 60 years; 56 males) with Hodgkin’s lymphoma (n=8) or non-Hodgkin’s lymphoma (n=86). Maximum standardized uptake values on the iliac crest of patients with lymphoma infiltrated bone marrow were significantly higher than those of patients with intact bone marrow (2.2±1.2 g/mL vs. 1.3±0.4 g/mL; p=0.001). The calculated values for FDG PET-CT during evaluation of bone marrow involvement were as follows: sensitivity 50%, specificity 96%, positive predictive value 80%, negative predictive value 85%, and positive likelihood ratio (LR+) 11.7. The value of LR+ was 16.0 in patients with aggressive subtypes of non-Hodgkin’s lymphoma (NHL).
Conclusion
FDG PET-CT could not replace bone marrow biopsy due to the low sensitivity of FDG PET-CT for detection of bone marrow infiltration in lymphoma patients. Conversely, FDG PET-CT had high specificity and LR+; therefore, it could be a useful tool for image-guided biopsy for lymphoma staging, especially for aggressive subtypes of NHL. In addition, unilateral bone marrow biopsy could be substituted for bilateral bone marrow biopsy in lymphoma patients with increased FDG uptake on any iliac crest.
Treatment and prognosis of lymphoma strongly depend on the stage of the disease at the time of diagnosis [1,2]. Bone marrow (BM) involvement is of crucial importance to staging of lymphoma since it signifies stage 4 disease and affects both treatment and prognosis [3,4]. BM biopsy is the established method for detection of BM involvement [5,6]; however, the value of unilateral versus bilateral BM biopsy remains controversial.
F-18 fluorodeoxyglucose positron emission tomography–computed tomography (FDG PET-CT) is noninvasive and semi-quantitative, and is the most specific and sensitive molecular imaging technique for staging and response evaluation of lymphoma [7,8]. FDG PET-CT was recently evaluated as a supplementary lymphoma staging method for BM involvement in several studies [9]; however, the question of whether it can reduce the need for BM biopsies at the iliac crest is still under discussion [10,11].
In this study, we assessed the ability of FDG PET-CT to ascertain the presence of BM involvement and define patients whose bilateral BM biopsies could be replaced by unilateral biopsy with PET-CT in lymphoma staging.
We retrospectively reviewed medical records of histologically proven lymphoma patients from 2004 through 2009 at the Hallym University Sacred Heart Hospital. The study design was approved by the institutional review board. BM biopsy was conducted on one side of the dorsal iliac crest and BM biopsy samples were analyzed following standard procedures, including formalin fixing and paraffin embedding and staining with hematoxylin and eosin. Additional immunohistochemical study was performed when necessary.
All data were acquired on a combined FDG PET-CT in line system (Discovery ST, General Electronic Medical Systems, Milwaukee, WI). Patients were studied after a 12-hour fast, and all had blood glucose levels < 160 mg/dL at the time of FDG injection. Whole body emission scans were acquired beginning at 60 minutes after an intravenous injection of FDG.
Pelvic bones were regarded as BM with hematopoietic activity during interpretation of FDG PET-CT scans. We used both qualitative (negative or positive) and semi-quantitative methods for evaluation of FDG PET-CT. Qualitative PET data were collected from retrospectively reviewed medical records, which were reported based on visual comparison of uptakes between the lesion and normal background by a nuclear medicine physician. The absence of tracer uptake or uptake less than or equal to liver parenchyma intensity in the BM sites was interpreted as ‘without a possibility’ of lymphomatous BM involvement by the nuclear medicine physician. Uptake of more than liver parenchyma intensity was interpreted as ‘with a possibility’ of involvement. Semi-quantitative PET data were calculated separately by the same nuclear medicine physician after the beginning of this study. All detected FDG-avid lesions in the iliac crest were objectively analyzed by measurement of the calculated maximum standardized uptake value (SUVmax), and the cut-off value for positivity was 2.0 g/mL, which was the median value of normal liver parenchyma with the settings of PET-CT.
Using a BM biopsy as the reference standard, we calculated the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR+) for FDG PET-CT during evaluation of BM infiltration.
An independent-samples Mann-Whitney U test was used to compare the mean scores of two groups on a given variable. The exact logistic regression was used to obtain the odds ratio. The exact confidence interval (CI) was calculated for sensitivity, specificity, PPV and NPV. Data were analyzed using SAS ver. 9.3 (SAS Institute, Cary, NC).
The study population included 94 patients (median age, 60 years; range, 20 to 80 years; 56 males) with Hodgkin’s lymphoma (HL, n=8) or non-Hodgkin’s lymphoma (NHL, n=86). Patient characteristics are listed in Table 1. Overall BM involvement, as confirmed by BM biopsy, occurred in 24 patients; HL (n=2, 25%), NHL (total, n=22, 26%; indolent NHL, n=4, 36%; moderately aggressive to aggressive NHL, n=18, 24%). BM involvement was odds 0.98 times greater in the NHL group than the HL group (95% CI, 0.30 to 2.44). The moderately aggressive to aggressive NHL group had odds 0.75 times as great as those of the indolent NHL group in BM involvement (95% CI, 0.35 to 1.70).
Mean SUVmax values on the iliac crest of patients with lymphoma infiltrated BM were significantly higher than those of patients with intact BM (2.2±1.2 g/mL vs. 1.3±0.4 g/mL, respectively; p=0.001). The results from subgroup analysis were as follows: HL with vs. without BM involvement, 2.0±0.4 g/mL vs. 1.4±0.3 g/mL, p=0.14; indolent NHL with vs. without BM involvement, 1.3±0.1 g/mL vs. 1.3±0.3 g/mL, p=1.0; moderately aggressive to aggressive NHL with vs. without BM involvement, 2.4±1.3 g/mL vs. 1.3±0.4 g/mL, p=0.001. In patients with BM involvement by lymphoma, values of SUVmax between indolent and moderately aggressive to aggressive NHL groups did not differ significantly (1.3±0.1 g/mL vs. 2.4±1.3 g/mL, p=0.08). Table 2 shows the detailed SUVmax values in each lymphoma subtype. The SUVmax in the extra-marrow site was 10.0±1.2 g/mL (95% CI, 7.7 to 12.4 g/mL) in patients with lymphoma infiltrated BM. Among these patients, the SUVmax of the extra-marrow sites were 6.0±2.0 g/mL in the indolent NHL group and 10.7±2.0 g/mL in the moderately aggressive to aggressive NHL groups (p=0.047).
Table 3 shows the calculated sensitivity, specificity, PPV, NPV values, and likelihood ratios for FDG PET-CT during evaluation of BM involvement measured based on a semiquantitative method. When compared with the semi-quantitative method in all patients (Fig. 1), values measured by the qualitative method were not significantly different (sensitivity, 54%; 95% CI, 33 to 74; specificity, 100%; 95% CI, 95 to 100; PPV, 100%; 95% CI, 75 to 100; NPV, 86%, 95% CI, 77 to 93).
BM involvement is one of the most important prognostic factors in patients with lymphomas. Therefore, BM biopsy with or without an aspirate is now included as part of the essential evaluation for initial staging in patients with lymphomas.
Conventional wisdom has long advocated the use of bilateral BM sampling for lymphoma staging with demonstrated improved detection rates of BM involvement. Brunning et al. [12] and Coller et al. [13] reported an increased yield of 10%-22% in bilateral biopsy specimens relative to unilateral specimens; therefore, bilateral BM biopsy is preferred to unilateral BM biopsy for staging evaluation of lymphoma. However, this is an invasive and painful diagnostic procedure that has side effects (infection, hemorrhage, trauma; 0.12%-0.30%) [14,15]. Moreover, reported rates of unilateral involvement in bilateral biopsies range from 10% to 50% because of the focal BM involvement pattern by lymphoma [16,17]. Recent data suggest that bilateral sampling is not mandatory if optimal demonstration of histological BM involvement will be obtained with a trephine biopsy from a single site of ≥20 mm in length [18]. However, obtaining adequate samples every time is difficult in a clinical setting. Accordingly, efforts to establish an alternative or at least a complementary diagnostic tool to demonstrate lymphomatous BM involvement should be undertaken. Several studies have defined the role of PET-CT in the detection of lymphomatous BM involvement [8,10]. As with a previous investigation of PET-CT (PPV 86%) [7], our data demonstrated that FDG PET-CT had a similar PPV (80%) for detection of BM infiltration in lymphoma patients. These findings suggests a new indication for FDG PET-CT for selection of patients who are candidates for unilateral BM biopsy.
Overall, BM involvement occurs in 30% to 50% of all patients with NHL, 40% to 90% of patients with indolent NHL and 18% to 36% of patients with aggressive NHL [19,20], which is in accordance with the results of the present study. Due to ethnic differences, the proportions of HL and follicular lymphoma patients in our study were small. In Korean and western studies, B-cell subtypes comprise the majority of cells in lymphoma, and diffuse large B-cell lymphoma is the most common subtype. Conversely, T-cell lymphoma is relatively common, while HL (6%) and follicular lymphoma (5%) are rare [21].
The uptake of FDG was correlated with the histologic grade of lymphoma and the proliferation rate of malignant cells [22]. It has been noted that some low grade NHL may have low or absent FDG uptake, which could limit the use of FDG PET-CT [23]. These results are concordant with those of meta-analysis by Pakos et al. [24]. Additionally, our findings were in accordance with those of previous studies, but the SUVmax values of extra-marrow were significantly lower in indolent subtypes than moderately aggressive to aggressive subtypes of NHL. Indolent subtypes had numerically lower SUVmax at the involved iliac crest than moderately aggressive to aggressive subtypes of NHL, but this difference was not significant. Our data showed that, in the HL and indolent NHL group, BM involvement by lymphoma did not influence FDG uptake on iliac crests. However, in moderately aggressive to aggressive subtypes of the NHL group, SUVmax values of involved marrow were significantly higher than those of uninvolved marrow (Fig. 2). These findings could be due to there being a small number of patients, especially HL and indolent lymphoma groups, or because of differences in the lymphoma involvement pattern between lymph node and BM. FDG PET-CT would not be useful to define BM involvement by HL or indolent lymphoma, but further large scale studies are needed to clarify these findings.
Meta-analysis revealed that the sensitivity rates of FDG PET-CT for identification of BM involvement ranged from 0% to 100%, while the respective specificity rates ranged from 72% to 100% [24]. Our data showed similar sensitivity (50%) and specificity (96%). With FDG PET-CT, false-positive results would be increased by other factors such as chemotherapy, infection, inflammation, hyperplastic marrow, and granulocyte colony-stimulating factor administration [25]. Use of biopsy reference standard may lead to a false negative result of FDG PET-CT during detection of BM infiltration by lymphoma. As noted in another trial [7], the present study showed that FDG PET-CT could not replace BM biopsy due to low sensitivity and that the negative result of FDG PET-CT could not rule out BM involvement by lymphoma. However, FDG-PET scans showed a high specificity rate of 96% and a LR+ value of 11.7. In the moderately aggressive to aggressive NHL group, areas of BM associated with disease would be 16 times more likely to have positive FDG uptake than intact BM sites. Therefore, unilateral BM biopsy of the FDG uptake site could be substituted for bilateral BM biopsy, especially in moderately aggressive to aggressive subtypes of NHL patients. Moreover, FDG PET-CT image-guided unilateral BM biopsy would reduce the number of painful procedures and side effects.
Our study is the first to compare semi-quantitative and qualitative analysis of FDG PET-CT for evaluation of BM involvement by lymphoma. Values between semi-quantitative and qualitative methods did not show a significant difference. Although the qualitative method is subjective, we suggest that, when analyzed by a well trained nuclear medicine physician, the qualitative method is positively correlated with the semi-quantitative method.
In conclusion, this study showed that FDG PET-CT had the potential for detection of BM involvement by lymphoma, especially in moderately aggressive or aggressive subtypes of NHL, and that it could be a useful tool for image-guided biopsy for lymphoma staging. In lymphoma patients with increased FDG uptake on any iliac crest, unilateral BM biopsy could be substituted for bilateral BM biopsy.
References
1. Jost LM, Stahel RA; ESMO Guidelines Task Force. ESMO Minimum clinical recommendations for diagnosis, treatment and follow-up of Hodgkin's disease. Ann Oncol. 2005; 16 Suppl 1:i54–5.

2. Hiddemann W, Dreyling M, Stahel RA; ESMO Guidelines Task Force. Minimum clinical recommendations for diagnosis, treatment and follow-up of newly diagnosed follicular lymphoma. Ann Oncol. 2005; 16 Suppl 1:i56–7.

3. Zhang QY, Foucar K. Bone marrow involvement by Hodgkin and non-Hodgkin lymphomas. Hematol Oncol Clin North Am. 2009; 23:873–902.

4. Juneja SK, Wolf MM, Cooper IA. Value of bilateral bone marrow biopsy specimens in non-Hodgkin's lymphoma. J Clin Pathol. 1990; 43:630–2.

5. Gronich N, Radnay J, Shapiro H, Manor Y, Lahav M, Lishner M. Clinical outcome of low-grade NHL patients with bone marrow involvement. Eur J Clin Invest. 2007; 37:305–9.

6. Sasaki M, Kuwabara Y, Koga H, Nakagawa M, Chen T, Kaneko K, et al. Clinical impact of whole body FDG-PET on the staging and therapeutic decision making for malignant lymphoma. Ann Nucl Med. 2002; 16:337–45.

7. Muslimani AA, Farag HL, Francis S, Spiro TP, Chaudhry AA, Chan VC, et al. The utility of 18-F-fluorodeoxyglucose positron emission tomography in evaluation of bone marrow involvement by non-Hodgkin lymphoma. Am J Clin Oncol. 2008; 31:409–12.

8. Schaefer NG, Strobel K, Taverna C, Hany TF. Bone involvement in patients with lymphoma: the role of FDG-PET/CT. Eur J Nucl Med Mol Imaging. 2007; 34:60–7.

9. Khan AB, Barrington SF, Mikhaeel NG, Hunt AA, Cameron L, Morris T, et al. PET-CT staging of DLBCL accurately identifies and provides new insight into the clinical significance of bone marrow involvement. Blood. 2013; 122:61–7.

10. Pelosi E, Penna D, Deandreis D, Chiappella A, Skanjeti A, Vitolo U, et al. FDG-PET in the detection of bone marrow disease in Hodgkin's disease and aggressive non-Hodgkin's lymphoma and its impact on clinical management. Q J Nucl Med Mol Imaging. 2008; 52:9–16.
11. Chen YK, Yeh CL, Tsui CC, Liang JA, Chen JH, Kao CH. F-18 FDG PET for evaluation of bone marrow involvement in non-Hodgkin lymphoma: a meta-analysis. Clin Nucl Med. 2011; 36:553–9.
12. Brunning RD, Bloomfield CD, McKenna RW, Peterson LA. Bilateral trephine bone marrow biopsies in lymphoma and other neoplastic diseases. Ann Intern Med. 1975; 82:365–6.

13. Coller BS, Chabner BA, Gralnick HR. Frequencies and patterns of bone marrow involvement in non-Hodgkin lymphomas: observations on the value of bilateral biopsies. Am J Hematol. 1977; 3:105–19.

14. Malempati S, Joshi S, Lai S, Braner DA, Tegtmeyer K. Videos in clinical medicine: bone marrow aspiration and biopsy. N Engl J Med. 2009; 361:e28.
15. Arellano-Rodrigo E, Real MI, Muntanola A, Burrel M, Rozman M, Fraire GV, et al. Successful treatment by selective arterial embolization of severe retroperitoneal hemorrhage secondary to bone marrow biopsy in post-polycythemic myelofibrosis. Ann Hematol. 2004; 83:67–70.

16. Moog F, Bangerter M, Kotzerke J, Guhlmann A, Frickhofen N, Reske SN. 18-F-fluorodeoxyglucose-positron emission tomography as a new approach to detect lymphomatous bone marrow. J Clin Oncol. 1998; 16:603–9.

17. Carr R, Barrington SF, Madan B, O'Doherty MJ, Saunders CA, van der Walt J, et al. Detection of lymphoma in bone marrow by whole-body positron emission tomography. Blood. 1998; 91:3340–6.

18. Campbell JK, Matthews JP, Seymour JF, Wolf MM, Juneja SK; Australasian Leukaemia Lymphoma Group. Optimum trephine length in the assessment of bone marrow involvement in patients with diffuse large cell lymphoma. Ann Oncol. 2003; 14:273–6.

19. Conlan MG, Bast M, Armitage JO, Weisenburger DD. Bone marrow involvement by non-Hodgkin's lymphoma: the clinical significance of morphologic discordance between the lymph node and bone marrow. Nebraska Lymphoma Study Group. J Clin Oncol. 1990; 8:1163–72.

20. Bennett JM, Cain KC, Glick JH, Johnson GJ, Ezdinli E, O'Connell MJ. The significance of bone marrow involvement in non-Hodgkin's lymphoma: the Eastern Cooperative Oncology Group experience. J Clin Oncol. 1986; 4:1462–9.

21. Kang YK, Kim BS, Kim TW, Ryu MH, Lee SS, Ryoo BY, et al. Clinicopathologic charcteristics of Korean non-Hodgkin's lymphomas based on REAL classification. J Korean Cancer Assoc. 1999; 31:641–52.
22. Okada J, Yoshikawa K, Itami M, Imaseki K, Uno K, Itami J, et al. Positron emission tomography using fluorine-18-fluorodeoxyglucose in malignant lymphoma: a comparison with proliferative activity. J Nucl Med. 1992; 33:325–9.
23. Goldberg MA, Lee MJ, Fischman AJ, Mueller PR, Alpert NM, Thrall JH. Fluorodeoxyglucose PET of abdominal and pelvic neoplasms: potential role in oncologic imaging. Radiographics. 1993; 13:1047–62.

24. Pakos EE, Fotopoulos AD, Ioannidis JP. 18F-FDG PET for evaluation of bone marrow infiltration in staging of lymphoma: a meta-analysis. J Nucl Med. 2005; 46:958–63.
Fig. 1.
Comparison of the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of fluorodeoxyglucose positron emission tomography– computed tomography during detection of bone marrow involvement between the semi-quantitative and qualitative method; 95% confidence intervals are displayed as a line.
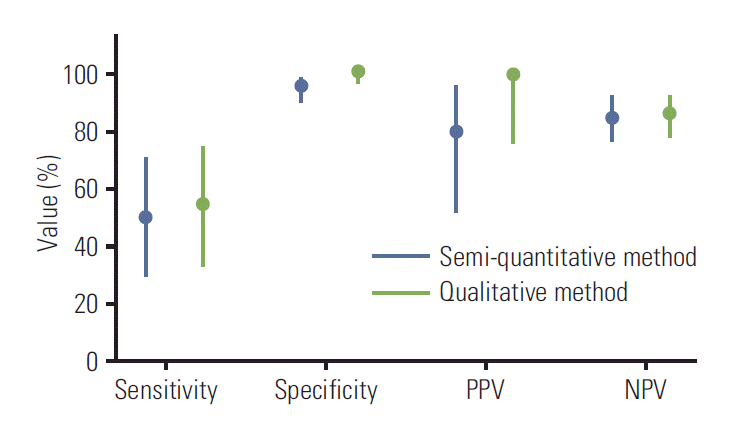
Fig. 2.
A 40-year-old female patient with angioimmunoblastic T-cell lymphoma. (A) Maximum intensity projection of positron emission tomography–computed tomography (PET-CT) shows multiple enlarged lymph nodes with increased fluorodeoxyglucose (FDG) uptake in the bilateral neck, axillar, retroperitoneal, iliac, and inguinal areas. Increased FDG uptakes in both lower lung fields suggest lung involvement. Transaxial PET (B) and fusion PET-CT (C) images demonstrate mild increased FDG uptake in bone marrow of iliac crests (arrows). The maximum standardized uptake value at the posterior iliac crest is 2.2 g/mL on the right and 2.4 g/mL on the left, respectively.
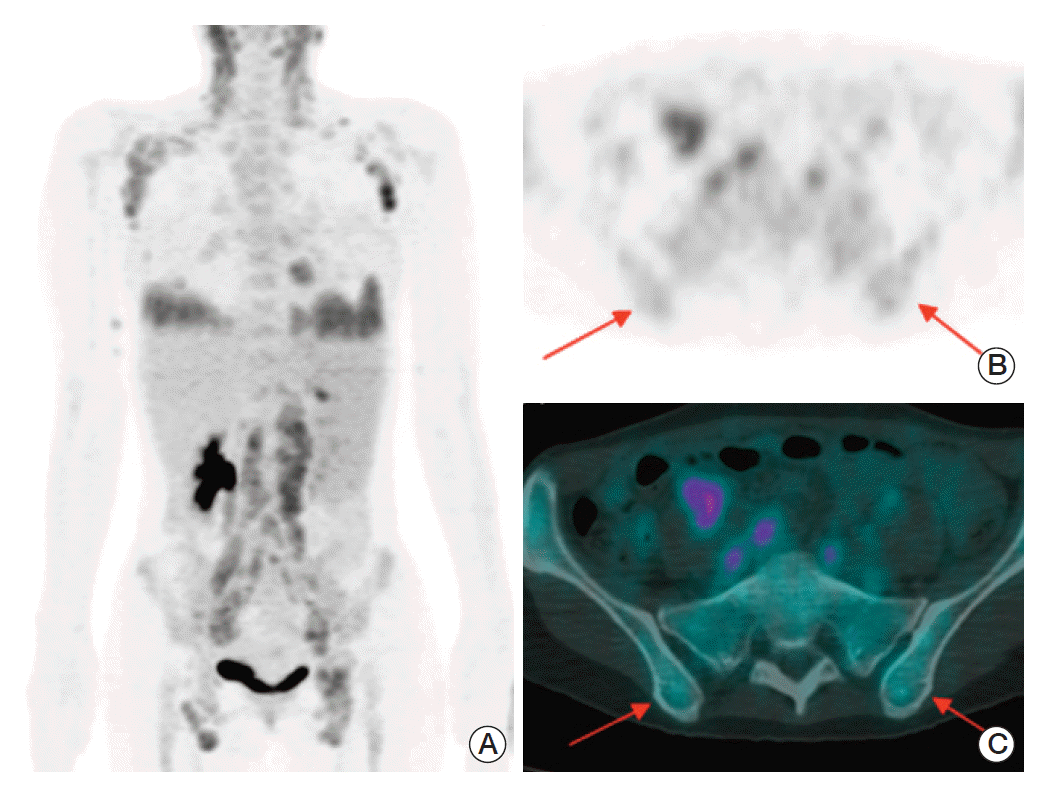
Table 1.
Patient characteristics (total group)
Table 2.
SUVmax values on iliac crests in patients with BM involvement by lymphoma
Table 3.
Calculated values of FDG PET-CT during evaluation of BM involvement by lymphoma
Values are presented as number (% or range). FDG PET-CT, fluorodeoxyglucose positron emission tomography–computed tomography; BM, bone marrow; HL, Hodgkin’s lymphoma; NHL, non-Hodgkin’s lymphoma; PPV, positive predictive value; NPV, negative predictive value; LR+, positive likelihood ratio (sensitivity/1-specificity); CI, confidence interval; NA, not available.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download