Abstract
Purpose
The purpose of this study is to investigate the current status of stereotactic body radiotherapy (SBRT) in Korea. A nationwide survey was conducted by the Korean Stereotactic Radiosurgery Group of the Korean Society for Radiation Oncology (KROG 13-13).
Materials and Methods
SBRT was defined as radiotherapy with delivery of a high dose of radiation to an extracranial lesion in ≤ 4 fractions. A 16-questionnaire survey was sent by e-mail to the chief of radiation oncology at 85 institutions in June 2013.
Results
All institutions (100%) responded to this survey. Of these, 38 institutions (45%) have used SBRT and 47 institutions (55%) have not used SBRT. Regarding the treatment site, the lung (92%) and liver (76%) were the two most common sites. The most common schedules were 60 Gy/4 fractions for non-small cell lung cancer, 48 Gy/4 fractions for lung metastases, 60 Gy/3 fractions for hepatocellular carcinoma, and 45 Gy/3 fractions or 40 Gy/4 fractions for liver metastases. Four-dimensional computed tomography (CT) was the most common method for planning CT (74%). During planning CT, the most common method of immobilization was the use of an alpha cradle/vacuum-lock (42%).
Since introduction of stereotactic radiosurgery (SRS) for treatment of arterio-venous malformation by Leksell in 1951 [1], SRS with delivery of a high dose of radiation in a single session has been used for treatment of lesions in the brain and spine [2]. Subsequently, stereotactic body radiotherapy (SBRT) was derived from SRS with improvements in radiation technology. SBRT is a “newly emerging treatment method to deliver a high dose of radiation to the target, utilizing either a single dose or a small number of fractions with a high degree of precision within the body” [3]. Many articles describing the promising results of SBRT have recently been published: most experience with SBRT involves treatment of lung cancer and liver metastases, whereas there has been less experience with hepatocellular carcinoma (HCC), and far less with pancreatic cancer and other malignancies [4].
Despite increased use of SBRT, little is known about the methodology and technology of SBRT in the radiation oncology community. To the best of our knowledge, nationwide surveys of SBRT have been conducted in few countries, however, no surveys have been conducted to assess the use of SBRT in Korea [5-9]. The indication of reimbursement for SBRT expended to include CyberKnife in 2011, since SBRT was approved by the National Health Insurance Service in 2003. Consequently, a number of institutions in Korea have considered a potential increase in the use of SBRT. Therefore, a nationwide survey on the use of SBRT in Korea was conducted by the Korean Stereotactic Radiosurgery Group of the Korean Society for Radiation Oncology.
In Korea, SBRT is covered by the National Health Insurance Service in cases of radiotherapy regimens using ≤ 4 fractions applied to lesions within the body. Therefore, for the survey, we defined SBRT as radiotherapy with delivery of a high dose of radiation to an extracranial lesion in ≤ 4 fractions. A 16-questionnaire survey was designed to determine the aspects of SBRT use, including treatment site, prescribed dose, moving organ control system, treatment machine, and planning system. Because the respondents were able to select multiple answers for certain questions, the total percentage did not add up to 100% for selected questions.
In 2013, 85 institutions in Korea have a department of radiation oncology. To assess the current status of SBRT use, we sent the survey by e-mail to the chief of radiation oncology of all 85 institutions in June 2013. The completed surveys were returned by e-mail after three weeks. In the event of non-response, the institutions were contacted by telephone several times in addition to being sent e-mails in order to achieve a 100% response rate. This study was conducted under the authorization and cooperation of the Korea Radiation Oncology Group (KROG 13-13).
Eighty-five institutions (100%) responded by June 2013. Of these, 38 institutions (45%) have used SBRT and 47 institutions (55%) have not used SBRT. Among those that have not used SBRT, 11 institutions (23%) have already been prepared to start SBRT in 2013. When the respondents were allowed up to two answers, the most common reason for the use of SBRT was the delivery of higher doses than that possible with conventional radiotherapy (89%). The next most common reasons were the shortening of treatment duration to improve patients’ convenience and reduce the mechanical load of the treatment machine (24%), and retreatment (21%). Additional reasons for use of SBRT were the shortening of treatment duration in order to start another treatment as early as possible when using a multidisciplinary approach (11%), participation in a clinical trial (8%), and the promising treatment outcomes of SBRT (3%). The most common reason for not using SBRT was the lack of special equipment (36%), followed by the lack of appropriate patients for SBRT (32%). Use of other fractionation such as hypofractionation (19%) and the lack of experience with use of SBRT (13%) were other reasons for not using SBRT.
In Korea, since introduction of SBRT at one institution in 1997, the number of institutions using SBRT has shown a gradual increase (Fig. 1A). According to this survey, we expect that 58% of institutions would use SBRT in 2014. As of June, 2013, the number of SBRT cases per year was ≥ 100 in three institutions (8%), 50-99 in five (13%), 30-49 in three (8%), 10-29 in 13 (34%), and < 10 in 14 (37%) (Fig. 1B). Regarding the treatment site, the lung (92%) and liver (76%) were the two most common sites treated with SBRT. Other treatment sites were the pancreas (21%), prostate (18%), retroperitoneum (18%), head and neck (18%), and adrenal glands (13%) (Fig. 2A). Cumulative adoption of SBRT for the two most common treatment sites, the lung and liver, is shown in Fig. 2B.
Prescribed doses for lung and liver SBRT varied among institutions, as shown in Fig. 3. The most common schedule for non-small cell lung cancer (NSCLC) was 60 Gy in four fractions (11 institutions, 32%), followed by 48 Gy in four fractions (nine institutions, 26%) and 60 Gy in three fractions (five institutions, 15%). The most common schedule for lung metastases was 48 Gy in four fractions (10 institutions, 35%), followed by 60 Gy in four fractions (seven institutions, 24%), and 60 Gy in three fractions (five institutions, 17%). The most common schedule for HCC was 60 Gy in three fractions (seven institutions, 26%), followed by 45 Gy in three fractions (four institutions, 15%), 48 Gy in four fractions (three institutions, 11%), and 40 Gy in four fractions (three institutions, 11%). The prescribed dose for treatment of liver metastases showed greater variety among institutions. The most common schedule was 45 Gy in three fractions or 40 Gy in four fractions (four institutions each, 19%), followed by 60 Gy in three fractions, or 48 or 60 Gy in four fractions (three institutions each, 14%).
The most common method of planning computed tomography (CT) for SBRT was to use four-dimensional CT (4DCT) (28 institutions, 74%), followed by inhalation and exhalationbreath-hold CT (nine institutions, 24%), and free-breathing CT (eight institutions, 21%). Otherwise, some institutions used slow CT (13%) or free-breathing CT combined with fluoroscopy (8%). The most common method of immobilization during planning CT was the use of an alpha cradle/vacuum-lock (16 institutions, 42%), followed by the use of a stereotactic body frame (10 institutions, 26%), wingboard (10 institutions, 26%), alpha cradle/vaccume-lock and wingboard (10 institutions, 26%), and so on. For control of respiratory motion, respiratory-gated radiotherapy with Real-Time Position Management (Varian Medical Systems, Palo Alto, CA) (24 institutions, 63%) and forced shallow breathing with abdominal compression (13 institutions, 34%) were the two most commonly used techniques. Details of the methods used for control of moving organs are summarized in Table 1.
The treatment machines used for SBRT varied among institutions. Thirteen institutions (34%) had ≥ 2 specially equipped treatment machines for SBRT and could select the suitable treatment machine according to treatment site and patients’ characteristics. The three most commonly used treatment machines were RapidArc (Varian Medical Systems) (16 institutions, 42%), Varian Clinac iX (Varian Medical Systems) (10 institutions, 26%), and CyberKnife (Accuray Inc., Sunnyvale, CA) (eight institutions, 21%) (Table 2). For SBRT planning, 15 institutions (39%) had ≥ 2 planning systems. The majority of SBRT users (27 institutions, 71%) used Eclipse (Varian Medical Systems). For the remaining users, eight (21%) used iPlan (BrainLAB AG, Feldkirchen, Germany), seven (18%) used the CyberKnife planning systems, six (16%) used the Pinnacle system (Philips, Milpitas, CA), four (11%) used a Tomotherapy Hi-Art or HD unit (Accuray Inc.), two (5%) used MONACO (Elekta, Crawley, UK), and one (3%) used Nucletron Oncentra MasterPlan (Nucletron BV, Veenendaal, The Netherlands). For optimal dose distribution for tumor and normal organ, 25 institutions (66%) used various planning techniques. The most common SBRT planning technique was static intensity-modulated radiotherapy (IMRT) (29 institutions, 76%), followed by dynamic conformal arc radiotherapy (19 institutions, 50%), 3-dimensional conformal radiotherapy with multiple beam arrangements (14 institutions, 37%), robotic SBRT (eight institutions, 21%), and rotational IMRT (six institutions, 16%).
As an assisted device for target localization, fiducials are rarely used in Korea. Insertion of fiducials is always performed in only one institution (3%), and fiducials are sometimes inserted in eight institutions (21%). For target localization before each treatment, the most common verification method was conebeam CT (33 institutions, 87%), followed by orthogonal kilovoltage radiography (19 institutions, 50%), orthogonal megavoltage localization image (six institutions, 16%), and fluoroscopy (four institutions, 11%). During treatment for a moving target, nearly half of the institutions (45%) did not use respiratory gating methods. Among users of respiratory gating methods, the most common application rate of gating treatment to moving targets was 10%-39% (seven institutions), as shown in Fig. 4.
In Korea, the use of SBRT was introduced in 1997 and has increased over time. Consistent with the results of surveys conducted in Japan and the United States, in Korea, the most common sites treated using SBRT were the lung and liver [5,6]. However, there is discordance between countries with regard to preferred prescribed doses. The most common schedule for lung cancer was 60 Gy in three fractions in the United States and 48 Gy in four fractions in Japan, whereas, in Korea, the most common schedule was 60 Gy in four fractions for primary lung cancer and 48 Gy in four fractions for lung metastases. In cases of liver SBRT, there is more variation in preferred prescribed doses between countries. In the United States, the most common schedule for liver cancer was 45 Gy in three fractions. In Japan, the most common schedule was 48 Gy in four fractions for primary liver cancer, and 50 Gy in five fractions or 48 Gy in four fractions for liver metastases, whereas, in Korea, the most common schedule was 60 Gy in three fractions for HCC, and 45 Gy in three fractions or 40 Gy in four fractions for liver metastases.
The optimal fraction and irradiated dose for SBRT remains unclear. Fortunately, there seems to be an emerging consensus on use of SBRT for treatment of NSCLC. A Japanese multi-institutional study concluded that a biologically effective dose (BED) of 100-150 Gy (α/β=10 Gy) was feasible and beneficial for stage I NSCLC [10]. Results of a meta-analysis suggested a BED of 83.2-146 Gy10, and the slight increase in toxicity for a BED of > 146 Gy10 was magnified because most patients who received SBRT were medically inoperable and relatively older age [11]. The largest cohort study conducted in this regard showed that the dose-response relationship for local control reached a plateau at a BED of 105 Gy10 [12]. Our survey showed that most institutions in Korea followed these guidelines. On the other hand, treatment of HCC using SBRT is currently under discussion, and phase I-II studies have been conducted with various prescribed doses. HCC is a radiosensitive cancer and has a dose-response relationship [13-15]. Several studies using SBRT with 30-60 Gy in 3-5 fractions showed 2- or 3-year local control rate of 68%-95% [16-19]. Ongoing phase II studies on the use of SBRT for HCC conducted in Korea would be helpful in drawing a proper conclusion regarding the fraction and irradiated dose (NCT01825824, NCT01850667, KCT0000625, and NCT01910909). Further studies on nationwide patterns-of-care and outcome analyses, based on our survey, would support the standardization of SBRT use for HCC in Korea.
According to results of our survey, in Korea, the most common method for planning CT was 4DCT (74%), similar to the findings of surveys conducted in the United States, and Germany and Austria [8,9]. However, the methods of immobilization used during planning CT differed among countries. Use of a stereotactic body frame was the most common in Japan (68%), whereas, an alpha cradle/vaccum-lock was the most commonly used in the United States (52%), followed by a stereotactic body frame (24%) [5,8]. In Korea, alpha cradle/vacuum-lock and stereotactic body frame were similarly used. These findings might be owing to the recent application of in-room image guidance. In Japan, a survey conducted in 2005 showed that the most common verification method before each SBRT was the use of a portal film. In contrast, surveys conducted in both the United States in 2010 and in Korea in 2013 showed that most institutions used in-room CT or planar images for target localization. Initially, a stereotactic body frame was developed for improvement of reproducibility and was regarded as an essential structural component for SBRT [20]. However, the advancements in image guidance ensure high geometric accuracy for moving organs irrespective of the immobilization method used, and, therefore, use of a stereotactic body frame is no longer essential [21]. The SBRT delivery technique also varied among countries. Three-dimensional conformal radiotherapy with multiple beam arrangements was the most commonly used delivery technique in Japan (79%), whereas, static IMRT was the most commonly used technique in Korea (76%). These factors are generally considered to affect the clinical results of SBRT. However, all of these uncertainties could be of smaller clinical relevance than previously estimated if a stereotactic ablative dose is delivered [9].
In Korea, SBRT is used at 38 institutions (45%), and its use is expected to show a continuous increase. However, SBRT practices vary among institutions. These findings underscore the need for additional prospective studies to determine the appropriate prescribed doses and to standardize the use of SBRT.
ACKNOWLEDGMENTS
This work was supported by the National Nuclear R & D program of the Ministry of Education, Science and Technology, Republic of Korea. And this work was supported by the Soonchunhyang University Research Fund.
References
1. Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951; 102:316–9.
2. Tipton K, Launders JH, Inamdar R, Miyamoto C, Schoelles K. Stereotactic body radiation therapy: scope of the literature. Ann Intern Med. 2011; 154:737–45.
3. Potters L, Steinberg M, Rose C, Timmerman R, Ryu S, Hevezi JM, et al. American Society for Therapeutic Radiology and Oncology and American College of Radiology practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2004; 60:1026–32.
4. Khrizman P, Small W Jr, Dawson L, Benson AB 3rd. The use of stereotactic body radiation therapy in gastrointestinal malignancies in locally advanced and metastatic settings. Clin Colorectal Cancer. 2010; 9:136–43.
5. Nagata Y, Hiraoka M, Mizowaki T, Narita Y, Matsuo Y, Norihisa Y, et al. Survey of stereotactic body radiation therapy in Japan by the Japan 3-D Conformal External Beam Radiotherapy Group. Int J Radiat Oncol Biol Phys. 2009; 75:343–7.
6. Pan H, Simpson DR, Mell LK, Mundt AJ, Lawson JD. A survey of stereotactic body radiotherapy use in the United States. Cancer. 2011; 117:4566–72.
7. Lock MI, Hoyer M, Bydder SA, Okunieff P, Hahn CA, Vichare A, et al. An international survey on liver metastases radiotherapy. Acta Oncol. 2012; 51:568–74.
8. Daly ME, Perks JR, Chen AM. Patterns-of-care for thoracic stereotactic body radiotherapy among practicing radiation oncologists in the United States. J Thorac Oncol. 2013; 8:202–7.
9. Guckenberger M, Allgauer M, Appold S, Dieckmann K, Ernst I, Ganswindt U, et al. Safety and efficacy of stereotactic body radiotherapy for stage 1 non-small-cell lung cancer in routine clinical practice: a patterns-of-care and outcome analysis. J Thorac Oncol. 2013; 8:1050–8.
10. Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004; 101:1623–31.
11. Zhang J, Yang F, Li B, Li H, Liu J, Huang W, et al. Which is the optimal biologically effective dose of stereotactic body radiotherapy for Stage I non-small-cell lung cancer? A meta-analysis. Int J Radiat Oncol Biol Phys. 2011; 81:e305–16.
12. Grills IS, Hope AJ, Guckenberger M, Kestin LL, Werner-Wasik M, Yan D, et al. A collaborative analysis of stereotactic lung radiotherapy outcomes for early-stage non-small-cell lung cancer using daily online cone-beam computed tomography image-guided radiotherapy. J Thorac Oncol. 2012; 7:1382–93.
13. Wigg AJ, Palumbo K, Wigg DR. Radiotherapy for hepatocellular carcinoma: systematic review of radiobiology and modeling projections indicate reconsideration of its use. J Gastroenterol Hepatol. 2010; 25:664–71.
14. Dawson LA, McGinn CJ, Normolle D, Ten Haken RK, Walker S, Ensminger W, et al. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2000; 18:2210–8.
15. Lausch A, Sinclair K, Lock M, Fisher B, Jensen N, Gaede S, et al. Determination and comparison of radiotherapy dose responses for hepatocellular carcinoma and metastatic colorectal liver tumours. Br J Radiol. 2013; 86:20130147.
16. Kwon JH, Bae SH, Kim JY, Choi BO, Jang HS, Jang JW, et al. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer. 2010; 10:475.
17. Louis C, Dewas S, Mirabel X, Lacornerie T, Adenis A, Bonodeau F, et al. Stereotactic radiotherapy of hepatocellular carcinoma: preliminary results. Technol Cancer Res Treat. 2010; 9:479–87.
18. Huang WY, Jen YM, Lee MS, Chang LP, Chen CM, Ko KH, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012; 84:355–61.
19. Kang JK, Kim MS, Cho CK, Yang KM, Yoo HJ, Kim JH, et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. 2012; 118:5424–31.
20. Lax I, Blomgren H, Naslund I, Svanstrom R. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol. 1994; 33:677–83.
21. Li W, Purdie TG, Taremi M, Fung S, Brade A, Cho BC, et al. Effect of immobilization and performance status on intrafraction motion for stereotactic lung radiotherapy: analysis of 133 patients. Int J Radiat Oncol Biol Phys. 2011; 81:1568–75.
Fig. 1.
(A) Cumulative adoption of stereotactic body radiotherapy (SBRT) after its introduction in 1997; green bar indicates institutions which have already been prepared to start SBRT in 2013. (B) The number of SBRT cases per year in each institution using SBRT as of June 2013.
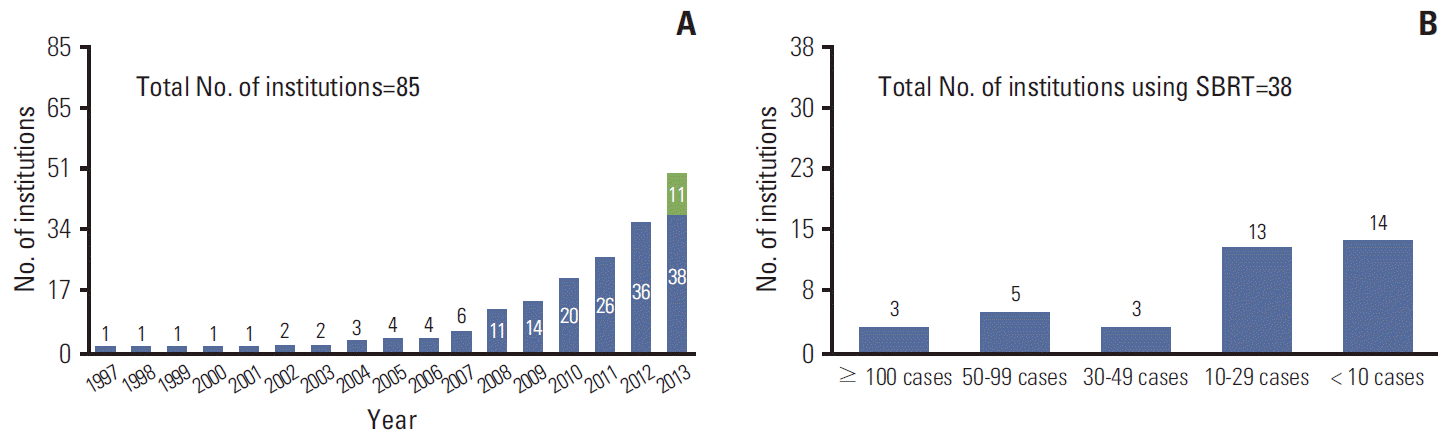
Fig. 2.
(A) Treatment sites applied stereotactic body radiotherapy (SBRT). (B) Cumulative adoption of SBRT for the two most common treatment sites (lung and liver).
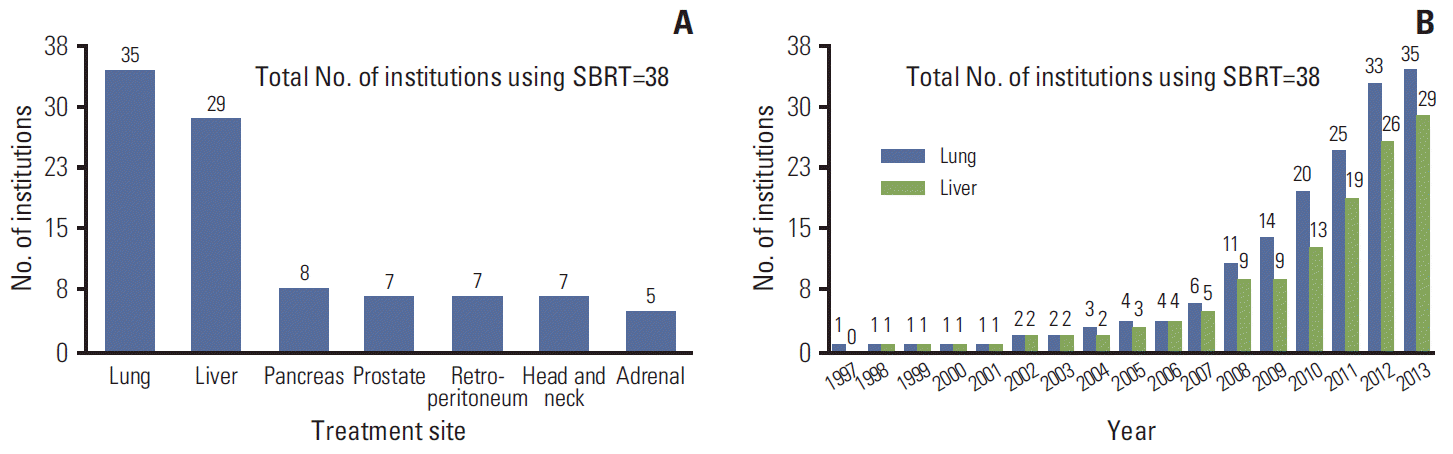
Fig. 3.
The prescribed doses for non-small cell lung cancer (A), lung metastases (B), hepatocellular carcinoma (C), and liver metastases (D) among institutions. SBRT, stereotactic body radiotherapy.
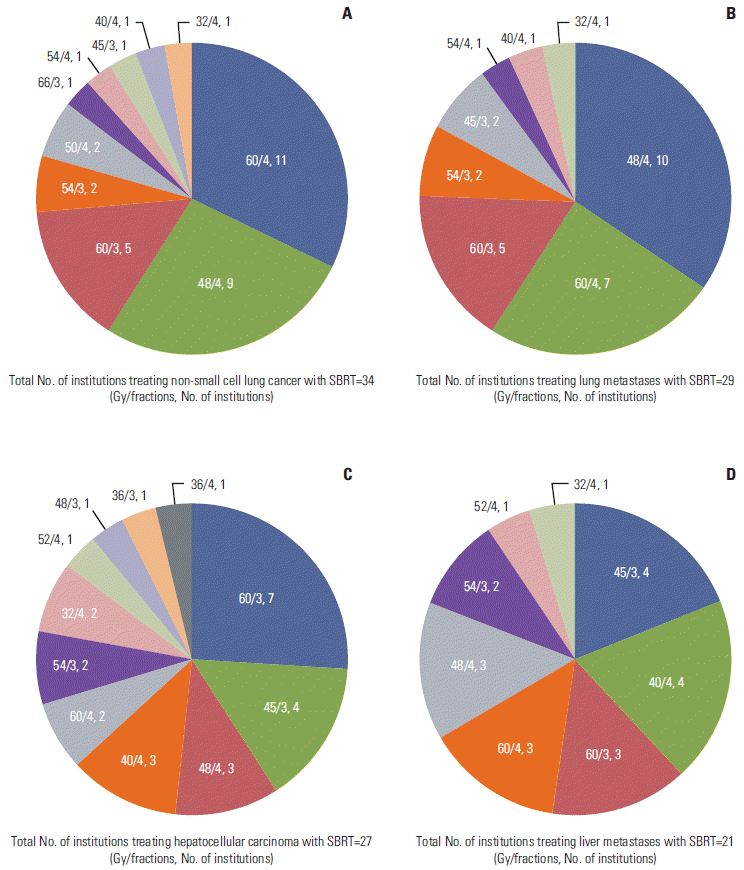
Fig. 4.
Application rate of respiratory gating method to moving targets according to institution. SBRT, stereotactic body radiotherapy.
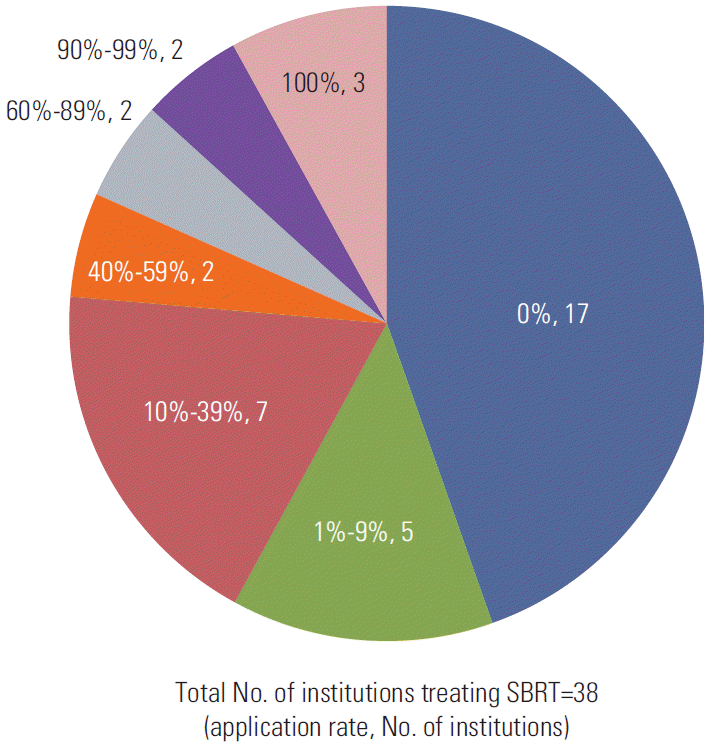
Table 1.
Details of the methods used for control of moving organs
Table 2.
Types of treatment machines for stereotactic body radiotherapy in Korea




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download